Surface-Templated Glycopolymer Nanopatterns Transferred to Hydrogels for Designed Multivalent Carbohydrate-Lectin Interactions across Length Scales
- PMID: 36640106
- PMCID: PMC9881003
- DOI: 10.1021/jacs.2c09937
Surface-Templated Glycopolymer Nanopatterns Transferred to Hydrogels for Designed Multivalent Carbohydrate-Lectin Interactions across Length Scales
Abstract
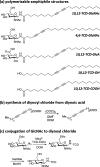
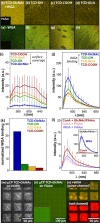
Multivalent interactions between carbohydrates and proteins enable a broad range of selective chemical processes of critical biological importance. Such interactions can extend from the macromolecular scale (1-10 nm) up to much larger scales across a cell or tissue, placing substantial demands on chemically patterned materials aiming to leverage similar interactions in vitro. Here, we show that diyne amphiphiles with carbohydrate headgroups can be assembled on highly oriented pyrolytic graphite (HOPG) to generate nanometer-resolution carbohydrate patterns, with individual linear carbohydrate assemblies up to nearly 1 μm, and microscale geometric patterns. These are then photopolymerized and covalently transferred to the surfaces of hydrogels. This strategy suspends carbohydrate patterns on a relatively rigid polydiacetylene (persistence length ∼ 16 nm), exposed at the top surface of the hydrogel above the bulk pore structure. Transferred patterns of appropriate carbohydrates (e.g., N-acetyl-d-glucosamine, GlcNAc) enable selective, multivalent interactions (KD ∼ 40 nM) with wheat germ agglutinin (WGA), a model lectin that exhibits multivalent binding with appropriately spaced GlcNAc moieties. WGA binding affinity can be further improved (KD ∼ 10 nM) using diacetylenes that shift the polymer backbone closer to the displayed carbohydrate, suggesting that this strategy can be used to modulate carbohydrate presentation at interfaces. Conversely, GlcNAc-patterned surfaces do not induce specific binding of concanavalin A, and surfaces patterned with glucuronic acid, or with simple carboxylic acid or hydroxyl groups, do not induce WGA binding. More broadly, this approach may have utility in designing synthetic glycan-mimetic interfaces with features from molecular to mesoscopic scales, including soft scaffolds for cells.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

