Withdrawal of infliximab or concomitant immunosuppressant therapy in patients with Crohn's disease on combination therapy (SPARE): a multicentre, open-label, randomised controlled trial
- PMID: 36640794
- PMCID: PMC9908559
- DOI: 10.1016/S2468-1253(22)00385-5
Withdrawal of infliximab or concomitant immunosuppressant therapy in patients with Crohn's disease on combination therapy (SPARE): a multicentre, open-label, randomised controlled trial
Abstract
Background: The combination of infliximab and immunosuppressant therapy is a standard management strategy for patients with Crohn's disease. Concerns regarding the implications of long-term combination therapy provided the rationale for a formal clinical trial of treatment de-escalation. Our aim was to compare the relapse rate and the time spent in remission over 2 years between patients continuing combination therapy and those stopping infliximab or immunosuppressant therapy.
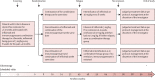
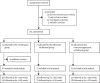
Methods: This multicentre, open-label, randomised controlled trial was performed in 64 hospitals in seven countries in Europe and Australia. Adult patients with Crohn's disease in steroid-free clinical remission for more than 6 months, on combination therapy of infliximab and immunosuppressant therapy for at least 8 months were randomly assigned (1:1:1) to either continue combination therapy (combination group), discontinue infliximab (infliximab withdrawal group), or discontinue immunosuppressant therapy (immunosuppressant withdrawal group). Randomisation was stratified according to disease duration before start of first anti-TNF treatment (≤2 or >2 years), failure of immunosuppressant therapy before start of infliximab, and presence of ulcers at baseline endoscopy. The patient number and group of each stratum were assigned by a central online randomisation website. Treatment was optimised or resumed in case of relapse in all groups. Participants, those assessing outcomes, and those analysing the data were not masked to group assignment. The coprimary endpoints were the relapse rate (superiority analysis) and time in remission over 2 years (non-inferiority analysis, non-inferiority margin 35 days). Analyses were done on an intention-to-treat basis. This study is registered with ClinicalTrials.gov, NCT02177071, and with EU Clinical Trials Register, EUDRACT 2014-002311-41. The trial was completed in April, 2021.
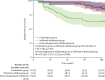
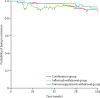
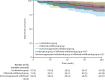
Findings: Between Nov 2, 2015, and April 24, 2019, 254 patients were screened. Of these, 211 were randomised and 207 were included in the final analysis (n=67 in the combination group, n=71 in the infliximab withdrawal group, and n=69 in the immunosuppressant withdrawal group). 39 patients had a relapse (eight [12%] of 67 in the combination group, 25 [35%] of 71 in the infliximab withdrawal group, six [9%] of 69 in the immunosuppressant withdrawal group). 2-year relapse rates were 14% (95% CI 4-23) in the combination group, 36% (24-47) in the infliximab withdrawal group, and 10% (2-18) in the immunosuppressant withdrawal group (hazard ratio [HR] 3·45 [95% CI 1·56-7·69], p=0·003, for infliximab withdrawal vs combination, and 4·76 [1·92-11·11], p=0·0004, for infliximab withdrawal vs immunosuppressant withdrawal). Of 28 patients who had a relapse and were retreated or optimised according to protocol, remission was achieved in 25 patients (one of two in the combination group, 22 of 23 in the infliximab withdrawal group, and two of three in the immunosuppressant withdrawal group). The mean time spent in remission over 2 years was 698 days (95% CI 668-727) in the combination group, 684 days (651-717) in the infliximab withdrawal group, and 706 days (682-730) in the immunosuppressant withdrawal group. The difference in restricted mean survival time in remission was -14 days (95% CI -56 to 27) between the infliximab withdrawal group and the combination group and -22 days (-62 to 16) between the infliximab withdrawal group and the immunosuppressant withdrawal group. The 95% CIs contained the non-inferiority threshold (-35 days). We recorded 31 serious adverse events, in 20 patients, with no difference in frequency between groups. The most frequent serious adverse events were infections (four in the combination group, two in the infliximab withdrawal group, and one in the immunosuppressant withdrawal group) and Crohn's disease exacerbation (three in the combination group, four in the infliximab withdrawal group, and one in the immunosuppressant withdrawal group). No death nor malignancy was recorded.
Interpretation: In patients with Crohn's disease in sustained steroid-free remission under combination therapy with infliximab and immunosuppressant therapy, withdrawal of infliximab should only be considered after careful assessment of risks and benefits for each patient, whereas withdrawal of immunosuppressant therapy could generally represent a preferable strategy when considering treatment de-escalation.
Funding: European Union's Horizon 2020.
Copyright © 2023 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests EL has received educational and research grants from Janssen, Pfizer, AbbVie, and Takeda, Fresenius-Kabi; speaker fees from AbbVie, Dr Falk Pharma, Ferring, Janssen, Pfizer, Celgene, Bristol Myers Squibb (BMS), Galapagos, and Takeda; advisory board fees for AbbVie, Celgene, Ferring, Janssen, BMS, Pfizer, Takeda, Gilead-Galapagos, Arena, and Elli Lilly; and consultancy fees from AbbVie. JS reports research support from European Commission Horizon 2020 programme. ND has received research grants from AbbVie, Janssen, and Takeda; speaker fees from AbbVie, Celltrion, Pfizer, Janssen, and Takeda; and advisory board fees from AbbVie, Dr Falk Pharma, and Janssen. BS has served as a consultant for AbbVie, Arena, BMS, Boehringer, CT Scout, Galapagos, Gilead, Janssen, Lilly, and PredictImmune; and has received speaker's fees from AbbVie, CED Service GmbH, Dr Falk Pharma, Ferring, Janssen, Materia Prima, Pfizer, and Lilly. GD'H reports consultancy activities for AbbVie, Agomab, Alimentiv, AstraZeneca, AM Pharma, AMT, Arena Pharmaceuticals, BMS, Boehringer Ingelheim, Celltrion, Eli Lilly, Exeliom Biosciences, Exo Biologics, Galapagos, Index Pharmaceuticals, Kaleido, Roche, Gilead, GlaxoSmithKline, Gossamerbio, Pfizer, Immunic, Johnson & Johnson, Origo, Polpharma, Procise Diagnostics, Prometheus laboratories, Prometheus Biosciences, Progenity, and Protagonist; speaker's bureau for AbbVie, Arena, Galapagos, Gilead, Pfizer, BMS, and Takeda; and fees for data monitoring board activities for Galapagos, AbbVie, Astrazeneca, and Seres Health. PB has received financial support for research from AbbVie, Amgen, Celltrion, Mylan, Pfizer and Takeda; lecture fees from AbbVie, Celltrion, Galapagos, Janssen, Lilly, Pentax, and Takeda; advisory board fees from AbbVie, Arena Pharmaceuticals, BMS, Celltrion, Dr Falk Pharma, Galapagos, Janssen, Lilly, Pentax, PSI-CRO, Roche, Takeda, and Tetrameros. LV has received fees from AbbVie, Amgen, Biogen, Mylan, Takeda, MSD, Janssen, Pfizer, Ferring, and Galapagos. PI has received honoraria for talking on behalf of AbbVie, BMS, Celgene, Celltrion, Dr Falk Pharma, Ferring, Galapagos, Gilead, MSD, Janssen, Lilly, Pfizer, Takeda, Tillotts, Sapphire Medical, Sandoz, Shire, and Warner Chilcott; and for acting in an advisory capacity to AbbVie, Arena, Boehringer-Ingelheim, BMS, Celgene, Celltrion, Connect Biopharma, Genentech, Gilead, Hospira, Janssen, Lilly, MSD, Pfizer, Pharmacosmos, Prometheus, Roche, Sandoz, Samsung Bioepis, Takeda, Topivert, VH2, Vifor Pharma, and Warner Chilcott. SV has received consulting or lecture fees for AbbVie, Amgen, Sandoz, Janssen, MSD, Pfizer, Celltrion, and Takeda. CAL has received grants from Genentech, AbbVie, Eli Lilly, Pfizer, Roche, UCB Biopharma, Sanofi Aventis, Biogen IDEC, Orion OYJ, and AstraZeneca; grants and personal fees from Janssen, Takeda, and Ferring; and personal fees from Dr Falk Pharma, outside the submitted work. FB has received grant or research support from AbbVie, Amgen, Janssen, and Takeda; honoraria from AbbVie, Dr Falk Pharma, Arena, Celgene, Mundipharma, Ferring, Vifor, Janssen, Merck Sharp & Dohme, Pfizer, Norgine, and Takeda. MN received board membership, consultancy, or lecture fees from AbbVie, Amgen, Arena, Biogen, CTMA, Celltrion, Ferring, Fresenius, Janssen, Mayoli-Spindler, MSD, Pfizer, Sandoz, and Takeda. MF received consulting or lecture fees for AbbVie, Amgen, Biogen, Gilead, Sandoz, Janssen, MSD, Pfizer, Ferring, Lilly, Tillots, Celltrion, Fresenius, Galapagos, and Takeda. CG received consulting or lecture fees from AbbVie, Amgen, Celltrion, Biogen, Fresenius, Gilead, Janssen, MSD, Mylan, Pfizer, Takeda, and Vifor. SB-H has received advisory board or consulting fees from AbbVie, Takeda, Janssen, Celltrion, Pfizer, GlaxoSmithKline, Ferring, Novartis, Roche, Gilead, NeoPharm, Predicta Med, Galmed, Medial Earlysign, and Eli Lilly, and research support from AbbVie, Takeda, Janssen, Celltrion, Pfizer, and Galmed. J-FC has received research grants from AbbVie, Pfizer, and Takeda; payment for lectures from AbbVie, Amgen, Pfizer, and Takeda; consulting fees from AbbVie, Amgen, Arena Pharmaceuticals, Boehringer Ingelheim, BMS, Celgene Corporation, Eli Lilly, Ferring Pharmaceuticals, Galmed Research, Genentech, GlaxoSmithKline, Janssen Pharmaceuticals, Kaleido Biosciences, Imedex, Immunic, Iterative Scopes, Merck, Novartis, Otsuka, PBM Capital, Pfizer, Protagonist Therapeutics, Sanofi, Takeda, TiGenix, and Vifor; and holds stock options in Intestinal Biotech Development. EH has received lecture fees from Takeda, Janssen, and BMS; and consultant or advisory board fees from AbbVie, Gilead, and Janssen. All other authors declare no competing interests.
Figures
Comment in
-
Treatment withdrawal in Crohn's disease: slowly becoming clearer.Lancet Gastroenterol Hepatol. 2023 Mar;8(3):200-201. doi: 10.1016/S2468-1253(22)00405-8. Epub 2023 Jan 11. Lancet Gastroenterol Hepatol. 2023. PMID: 36640793 No abstract available.
-
Discontinuation of infliximab in patients with Crohn's disease on combination therapy.Nat Rev Gastroenterol Hepatol. 2023 Mar;20(3):131. doi: 10.1038/s41575-023-00751-w. Nat Rev Gastroenterol Hepatol. 2023. PMID: 36755080 No abstract available.
-
Treatment de-escalation in Crohn's disease.Lancet Gastroenterol Hepatol. 2023 May;8(5):401. doi: 10.1016/S2468-1253(23)00073-0. Lancet Gastroenterol Hepatol. 2023. PMID: 37030299 No abstract available.
-
Treatment de-escalation in Crohn's disease - Author's reply.Lancet Gastroenterol Hepatol. 2023 May;8(5):401-402. doi: 10.1016/S2468-1253(23)00081-X. Lancet Gastroenterol Hepatol. 2023. PMID: 37030300 No abstract available.
References
-
- Torres J, Mehandru S, Colombel J-F, Peyrin-Biroulet L. Crohn's disease. Lancet. 2017;389:1741–1755. - PubMed
-
- Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: an update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) initiative of the international organization for the study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. 2021;160:1570–1583. - PubMed
-
- Colombel JF, Panaccione R, Bossuyt P, et al. Effect of tight control management on Crohn's disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet. 2017;390:2779–2789. - PubMed
-
- Khanna R, Bressler B, Levesque BG, et al. Early combined immunosuppression for the management of Crohn's disease (REACT): a cluster randomised controlled trial. Lancet. 2015;386:1825–1834. - PubMed
-
- Rutgeerts P, D'Haens G, Targan S, et al. Efficacy and safety of retreatment with anti-tumor necrosis factor antibody (infliximab) to maintain remission in Crohn's disease. Gastroenterology. 1999;117:761–769. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

