Presynaptic Gq-coupled receptors drive biphasic dopamine transporter trafficking that modulates dopamine clearance and motor function
- PMID: 36640864
- PMCID: PMC9943899
- DOI: 10.1016/j.jbc.2023.102900
Presynaptic Gq-coupled receptors drive biphasic dopamine transporter trafficking that modulates dopamine clearance and motor function
Abstract
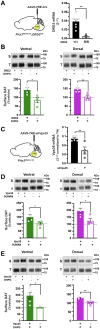
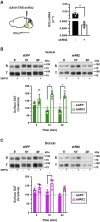
Extracellular dopamine (DA) levels are constrained by the presynaptic DA transporter (DAT), a major psychostimulant target. Despite its necessity for DA neurotransmission, DAT regulation in situ is poorly understood, and it is unknown whether regulated DAT trafficking impacts dopaminergic signaling and/or behaviors. Leveraging chemogenetics and conditional gene silencing, we found that activating presynaptic Gq-coupled receptors, either hM3Dq or mGlu5, drove rapid biphasic DAT membrane trafficking in ex vivo striatal slices, with region-specific differences between ventral and dorsal striata. DAT insertion required D2 DA autoreceptors and intact retromer, whereas DAT retrieval required PKC activation and Rit2. Ex vivo voltammetric studies revealed that DAT trafficking impacts DA clearance. Furthermore, dopaminergic mGlu5 silencing elevated DAT surface expression and abolished motor learning, which was rescued by inhibiting DAT with a subthreshold CE-158 dose. We discovered that presynaptic DAT trafficking is complex, multimodal, and region specific, and for the first time, we identified cell autonomous mechanisms that govern presynaptic DAT tone. Importantly, the findings are consistent with a role for regulated DAT trafficking in DA clearance and motor function.
Keywords: dopamine; membrane trafficking; metabotropic glutamate receptor; motor function; striatum.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures







References
-
- Wise R.A. Dopamine, learning and motivation. Nat. Rev. Neurosci. 2004;5:483–494. - PubMed
-
- Iversen S.D., Iversen L.L. Dopamine: 50 years in perspective. Trends Neurosci. 2007;30:188–193. - PubMed
-
- Hyman S.E., Malenka R.C., Nestler E.J. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu. Rev. Neurosci. 2006;29:565–598. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

