Genetically engineered human pituitary corticotroph tumor organoids exhibit divergent responses to glucocorticoid receptor modulators
- PMID: 36640905
- PMCID: PMC11345864
- DOI: 10.1016/j.trsl.2023.01.002
Genetically engineered human pituitary corticotroph tumor organoids exhibit divergent responses to glucocorticoid receptor modulators
Abstract
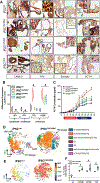
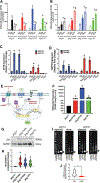
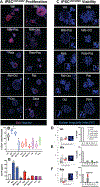
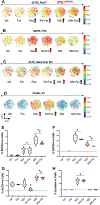
Cushing's disease (CD) is a serious endocrine disorder attributed to an adrenocorticotropic hormone (ACTH)-secreting pituitary neuroendocrine tumor (PitNET) that that subsequently leads to chronic hypercortisolemia. PitNET regression has been reported following treatment with the investigational selective glucocorticoid receptor (GR) modulator relacorilant, but the mechanisms behind that effect remain unknown. Human PitNET organoid models were generated from induced human pluripotent stem cells (iPSCs) or fresh tissue obtained from CD patient PitNETs (hPITOs). Genetically engineered iPSC derived organoids were used to model the development of corticotroph PitNETs expressing USP48 (iPSCUSP48) or USP8 (iPSCUSP8) somatic mutations. Organoids were treated with the GR antagonist mifepristone or the GR modulator relacorilant with or without somatostatin receptor (SSTR) agonists pasireotide or octreotide. In iPSCUSP48 and iPSCUSP8 cultures, mifepristone induced a predominant expression of SSTR2 with a concomitant increase in ACTH secretion and tumor cell proliferation. Relacorilant predominantly induced SSTR5 expression and tumor cell apoptosis with minimal ACTH induction. Hedgehog signaling mediated the induction of SSTR2 and SSTR5 in response to mifepristone and relacorilant. Relacorilant sensitized PitNET organoid responsiveness to pasireotide. Therefore, our study identified the potential therapeutic use of relacorilant in combination with somatostatin analogs and demonstrated the advantages of relacorilant over mifepristone, supporting its further development for use in the treatment of Cushing's disease patients.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of Interest All authors have read the journal's policy on disclosure of potential conflicts of interest.. Dr. Andreas G. Moraitis and Dr. Andrew E.Greenstein are employees and stock holders of Corcept. Dr. Kevin CJ Yuen has received research grants to the Barrow Neurological Institute from Crinetics, Ascendis, Corcept, and Amryt. Dr. Yuen has served as an advisory board member for Novo Nordisk, Ipsen, Amryt, Crinetics, Recordati and Xeris, and served as a speaker for Recordati, Novo Nordisk and Corcept. All other authors have no potential conflicts of interest to declare.
Figures




Similar articles
-
Glucocorticoid Receptor Antagonism Upregulates Somatostatin Receptor Subtype 2 Expression in ACTH-Producing Neuroendocrine Tumors: New Insight Based on the Selective Glucocorticoid Receptor Modulator Relacorilant.Front Endocrinol (Lausanne). 2022 Jan 4;12:793262. doi: 10.3389/fendo.2021.793262. eCollection 2021. Front Endocrinol (Lausanne). 2022. PMID: 35058882 Free PMC article.
-
Changes in plasma ACTH levels and corticotroph tumor size in patients with Cushing's disease during long-term treatment with the glucocorticoid receptor antagonist mifepristone.J Clin Endocrinol Metab. 2014 Oct;99(10):3718-27. doi: 10.1210/jc.2014-1843. Epub 2014 Jul 11. J Clin Endocrinol Metab. 2014. PMID: 25013998 Free PMC article. Clinical Trial.
-
Inhibitory effects of SOM230 on adrenocorticotropic hormone production and corticotroph tumor cell proliferation in vitro and in vivo.Mol Cell Endocrinol. 2014 Aug 25;394(1-2):37-46. doi: 10.1016/j.mce.2014.07.001. Epub 2014 Jul 8. Mol Cell Endocrinol. 2014. PMID: 25011056
-
The Role of Glucocorticoid Receptor in the Pathophysiology of Pituitary Corticotroph Adenomas.Int J Mol Sci. 2022 Jun 9;23(12):6469. doi: 10.3390/ijms23126469. Int J Mol Sci. 2022. PMID: 35742910 Free PMC article. Review.
-
The role of somatostatin analogs in Cushing's disease.Pituitary. 2004;7(4):257-64. doi: 10.1007/s11102-005-1404-x. Pituitary. 2004. PMID: 16132202 Review.
Cited by
-
Three Dimensional Models of Endocrine Organs and Target Tissues Regulated by the Endocrine System.Cancers (Basel). 2023 Sep 17;15(18):4601. doi: 10.3390/cancers15184601. Cancers (Basel). 2023. PMID: 37760571 Free PMC article. Review.
-
Endocrine cancer organoids in basic and translational medical research.Sci China Life Sci. 2025 Jun 5. doi: 10.1007/s11427-024-2888-8. Online ahead of print. Sci China Life Sci. 2025. PMID: 40488953 Review.
-
Organoid models of the pituitary gland in health and disease.Front Endocrinol (Lausanne). 2023 Aug 8;14:1233714. doi: 10.3389/fendo.2023.1233714. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37614709 Free PMC article. Review.
-
Advancements in Molecular Diagnosis and Pharmacotherapeutic Strategies for Invasive Pituitary Adenomas.Immun Inflamm Dis. 2024 Dec;12(12):e70098. doi: 10.1002/iid3.70098. Immun Inflamm Dis. 2024. PMID: 39688352 Free PMC article. Review.
-
The hidden hedgehog of the pituitary: hedgehog signaling in development, adulthood and disease of the hypothalamic-pituitary axis.Front Endocrinol (Lausanne). 2023 Jul 5;14:1219018. doi: 10.3389/fendo.2023.1219018. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37476499 Free PMC article. Review.
References
-
- Cushing H. The basophil adenomas of the pituitary body and their clinical manifestations (pituitary basophilism). Obes Res 1932;2:486–508. - PubMed
-
- Ironside N, Chen CJ, Lee CC, Trifiletti DM, Vance ML, Sheehan JP. Outcomes of Pituitary Radiation for Cushing’s Disease. Endocrinol Metab Clin North Am 2018;47:349–65. - PubMed
-
- Loriaux DL. Diagnosis and differential diagnosis of Cushing’s Syndrome. N Engl J Med 2017;377:e3. - PubMed
-
- Asa SL, Mete O, Perry A, Osamura RY. Overview of the 2022 WHO Classification of Pituitary Tumors. Endocr Pathol 2022;33:6–26. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

