Pharmacokinetics of dose-adjusted levonorgestrel emergency contraception combined with efavirenz-based antiretroviral therapy or rifampicin-containing tuberculosis regimens
- PMID: 36641094
- PMCID: PMC10187685
- DOI: 10.1016/j.contraception.2023.109951
Pharmacokinetics of dose-adjusted levonorgestrel emergency contraception combined with efavirenz-based antiretroviral therapy or rifampicin-containing tuberculosis regimens
Abstract
Objectives: To determine if double-dose levonorgestrel emergency contraception (EC) in combination with efavirenz or rifampicin, 2 drugs known to decrease levonorgestrel exposure, resulted in similar pharmacokinetics compared to standard-dose levonorgestrel EC without drug-drug interactions.
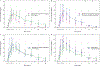
Study design: We conducted a phase 2, open-label, multicenter, partially randomized, 4 parallel group trial in pre-menopausal females ≥16 years old without an indication for EC and not on hormonal contraception. Participants on dolutegravir-based antiretroviral therapy (ART) received levonorgestrel 1.5 mg (control group); those on rifampicin-containing tuberculosis therapy received levonorgestrel 3 mg; those on efavirenz-based ART were randomized 1:2 to levonorgestrel 1.5 mg or 3 mg. Plasma was collected through 48 hours post-dose to assess levonorgestrel pharmacokinetics. Area under the concentration-time curve (AUC) over 8 hours was the primary outcome. Levonorgestrel pharmacokinetic parameters were compared between groups using geometric mean ratios (GMR) with 90% confidence intervals.
Results: The median (Q1, Q3) age for all participants (n = 118) was 34 (27, 41) years and BMI was 23.2 (20, 26.3) kg/m2. Participants receiving levonorgestrel 1.5mg plus efavirenz (n = 17) had 50% lower AUC0-8h compared to the control group (n = 32) [0.50 (0.40, 0.62)]. Participants receiving levonorgestrel 3 mg had a similar AUC0-8h when receiving either efavirenz (n = 35) [0.99 (0.81, 1.20)] or rifampicin (n = 34) [1.16 (0.99, 1.36)] compared to control. Levonorgestrel 3 mg resulted in similar or higher maximum concentration with either efavirenz [1.17 (0.96, 1.41)] or rifampicin [1.27 (1.09, 1.49)] compared to the control group.
Conclusions: Doubling the dose of levonorgestrel EC successfully increased levonorgestrel exposure over the first 8 hours in participants receiving either efavirenz-based ART or rifampicin-containing tuberculosis therapy.
Implications: Adjusting levonorgestrel emergency contraception from 1.5 mg to 3 mg improves levonorgestrel pharmacokinetic exposure in participants receiving either efavirenz-based antiretroviral regimens or rifampicin-containing tuberculosis therapy. These data support guideline recommendations to double the dose of levonorgestrel emergency contraception in persons on medications that decrease levonorgestrel exposure by inducing levonorgestrel metabolism.
Keywords: Drug interaction; Efavirenz; Emergency contraception; Pharmacokinetics; Rifampicin.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
DECLARATIONS OF INTEREST
KKS has received investigator-initiated research support from Organon, LLC, paid to her institution. All other authors report no declarations.
Figures

References
-
- Pillay T, Sturm AW, Khan M, Adhikari M, Moodley J, Connolly C, et al. Vertical transmission of Mycobacterium tuberculosis in KwaZulu Natal: impact of HIV-1 co-infection. Int J Tuberc Lung Dis. 2004;8:59–69. - PubMed
-
- Sobhy S, Babiker Z, Zamora J, Khan KS, Kunst H. Maternal and perinatal mortality and morbidity associated with tuberculosis during pregnancy and the postpartum period: a systematic review and meta-analysis. BJOG. 2017;124:727–33. - PubMed
-
- Curtis KM, Tepper NK, Jatlaoui TC, Berry-Bibee E, Horton LG, Zapata LB, et al. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1–103. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UM1 AI069476/AI/NIAID NIH HHS/United States
- U01 AI069476/AI/NIAID NIH HHS/United States
- UM1 AI069471/AI/NIAID NIH HHS/United States
- UL1 TR002489/TR/NCATS NIH HHS/United States
- UM1 AI068634/AI/NIAID NIH HHS/United States
- UL1 TR001422/TR/NCATS NIH HHS/United States
- UM1 AI069518/AI/NIAID NIH HHS/United States
- UM1 AI069453/AI/NIAID NIH HHS/United States
- UM1 AI069463/AI/NIAID NIH HHS/United States
- UM1 AI106701/AI/NIAID NIH HHS/United States
- UM1 AI069521/AI/NIAID NIH HHS/United States
- UM1 AI069419/AI/NIAID NIH HHS/United States
- R01 HD085887/HD/NICHD NIH HHS/United States
- UM1 AI069494/AI/NIAID NIH HHS/United States
- UM1 AI069423/AI/NIAID NIH HHS/United States
- UM1 AI069456/AI/NIAID NIH HHS/United States
- UL1 TR001857/TR/NCATS NIH HHS/United States
- U01 AI069463/AI/NIAID NIH HHS/United States
- UM1 AI069432/AI/NIAID NIH HHS/United States
- UM1 AI069399/AI/NIAID NIH HHS/United States
- U01 AI068636/AI/NIAID NIH HHS/United States
- UL1 TR002384/TR/NCATS NIH HHS/United States
- U01 AI069399/AI/NIAID NIH HHS/United States
- U01 AI069471/AI/NIAID NIH HHS/United States
- UM1 AI068636/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials

