Dentin primer based on a highly functionalized gelatin-methacryloyl hydrogel
- PMID: 36641338
- PMCID: PMC11391902
- DOI: 10.1016/j.dental.2022.12.005
Dentin primer based on a highly functionalized gelatin-methacryloyl hydrogel
Abstract
Gelatin-methacryloyl hydrogels (GelMA) have demonstrated their utility as scaffolds in a variety of tissue engineering applications.
Objectives: In this study, a highly functionalized GelMA hydrogel was synthesized and assessed for degree of functionalization. As the proposed GelMA hydrogel was coupled to a visible-light photoinitiator, we hypothesized it might serve as base to formulate a model dentin primer for application in restorative dentistry.
Methods: GelMA was mixed with photoinitiator lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP), photopolymerized for 0-40 s using a dental light-curing device and tested for extrudability, degree of photo-crosslinking (DPxlink), water sorption/solubility/swelling (WS/SL/SW) and apparent modulus of elasticity (AE). Model dentin primer was prepared by mixing GelMA+LAP with a primer of a commercial three-step etch-and-rinse adhesive. After application of GelMA-based primer to acid-etched dentin, samples were bonded with correspondent adhesive agent, photopolymerized and had their immediate bond strength compared to control samples primed and bonded with the same commercial material.
Results: Extrudability of hydrogel was confirmed using a microsyringe to write the acronym "CDMI". DPxlink of GelMA+LAP changed significantly as a function of photopolymerization time (20 s < 30 s ≤ 40 s). WS, SL and SW were significantly reduced in hydrogels polymerized for 30 and 40 s. AE of hydrogels varied significantly as a function of photopolymerization time (20 s < 30 s ≤ 40 s; 20 s ‡ 40 s). Bond strength of dentin primed with GelMA-based primer was lower (∼29.3 MPa) but not significantly of that of control (∼34.6 MPa).
Conclusions: Optimization of a GelMA-based dentin primers can lead to the development of promising biomimetic adhesives for dentin rehabilitation.
Keywords: Dentin; Extracellular matrix; Gelatin; Hydrogels; Methacrylamides; Model bonding agents.
Copyright © 2022 The Academy of Dental Materials. Published by Elsevier Inc. All rights reserved.
Figures
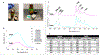


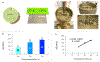

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

