Modulation of the gut microbiota engages antigen cross-presentation to enhance antitumor effects of CAR T cell immunotherapy
- PMID: 36641624
- PMCID: PMC10014349
- DOI: 10.1016/j.ymthe.2023.01.012
Modulation of the gut microbiota engages antigen cross-presentation to enhance antitumor effects of CAR T cell immunotherapy
Abstract
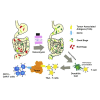
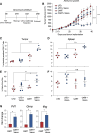
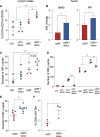
Several studies have shown the influence of commensal microbes on T cell function, specifically in the setting of checkpoint immunotherapy for cancer. In this study, we investigated how vancomycin-induced gut microbiota dysbiosis affects chimeric antigen receptor (CAR) T immunotherapy using multiple preclinical models as well as clinical correlates. In two murine tumor models, hematopoietic CD19+-A20 lymphoma and CD19+-B16 melanoma, mice receiving vancomycin in combination with CD19-directed CAR T cell (CART-19) therapy displayed increased tumor control and tumor-associated antigens (TAAs) cross-presentation compared with CART-19 alone. Fecal microbiota transplant from human healthy donors to pre-conditioned mice recapitulated the results obtained in naive gut microbiota mice. Last, B cell acute lymphoblastic leukemia patients treated with CART-19 and exposed to oral vancomycin showed higher CART-19 peak expansion compared with unexposed patients. These results substantiate the role of the gut microbiota on CAR T cell therapy and suggest that modulation of the gut microbiota using vancomycin may improve outcomes after CAR T cell therapy across tumor types.
Keywords: CAR T cell; antigen cross-presentation; gut microbiota; immunotherapy; vancomycin.
Copyright © 2023. Published by Elsevier Inc.
Conflict of interest statement
Declaration of interests M.R.: BMS, BAYER, GSK, consultancy; Novartis, patents and royalties; AbClon, consultancy, research funding; Tmunity, patents and royalties; viTToria Biotherapeutics, research funding. N.F.: Sana Biotechnology, consultancy; Novartis, research funding; Kite Pharma, consultancy; Syndax Pharmaceuticals, consultancy. C.H.J.: Tmunity, DeCART, BluesphereBio, Carisma, Cellares, Celldex, Cabaletta, Poseida, Verismo, and Ziopharm, current equity holder in publicly traded company; AC Immune, DeCART, BluesphereBio, Carisma, Cellares, Celldex, Cabaletta, Poseida, Verismo, Ziopharm, consultancy; Novartis, patents and royalties. D.L.P.: American Society for Transplantation and Cellular Therapy, honoraria; ASH and DeCart, membership on the Board of Directors or advisory committee; Genentech, current employment, current equity holder in publicly traded company; Incyte and Janssen, Kite/Gilead, and National Marrow Donor Program, membership on an entity’s board of directors or advisory committee; Novartis, membership on an entity’s board of directors or advisory committee, patents and royalties, and research funding; Unity, patents and royalties; and Wiley and Sons Publishing, honoraria. S.J.S.: TG Therapeutics, research funding; Incyte, research funding; Adaptive Biotechnologies, research funding; Pharmacyclics, research funding; Merck, research funding; Genentech/Roche, consultancy, research funding; Tessa Therapeutics, consultancy; Loxo Oncology, consultancy; Juno Therapeutics, consultancy, research funding; BeiGene, consultancy; Alimera Sciences, consultancy; Acerta Pharma/AstraZeneca, consultancy; Novartis, consultancy, honoraria, patents and royalties, research funding; AbbVie, consultancy, research funding; Nordic Nanovector, consultancy; Celgene, consultancy, honoraria, research funding.
Figures





References
-
- Maude S.L., Laetsch T.W., Buechner J., Rives S., Boyer M., Bittencourt H., Bader P., Verneris M.R., Stefanski H.E., Myers G.D., et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N. Engl. J. Med. 2018;378:439–448. doi: 10.1056/NEJMoa1709866. - DOI - PMC - PubMed
-
- Neelapu S.S., Locke F.L., Bartlett N.L., Lekakis L.J., Miklos D.B., Jacobson C.A., Braunschweig I., Oluwole O.O., Siddiqi T., Lin Y., et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N. Engl. J. Med. 2017;377:2531–2544. doi: 10.1056/NEJMoa1707447. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

