Methodological advancements in organ-specific ectopic lipid quantitative characterization: Effects of high fat diet on muscle and liver intracellular lipids
- PMID: 36642092
- PMCID: PMC9938329
- DOI: 10.1016/j.molmet.2023.101669
Methodological advancements in organ-specific ectopic lipid quantitative characterization: Effects of high fat diet on muscle and liver intracellular lipids
Abstract
Objective: Ectopic lipid accumulation is a hallmark of metabolic diseases, linking obesity to non-alcoholic fatty liver disease, insulin resistance and diabetes. The use of zebrafish as a model of obesity and diabetes is raising due to the conserved properties of fat metabolism between humans and zebrafish, the homologous genes regulating lipid uptake and transport, the implementation of the '3R's principle and their cost-effectiveness. To date, a method allowing the conservation of lipid droplets (LDs) and organs in zebrafish larvae to image ectopic lipids is not available. Our objectives were to develop a novel methodology to quantitatively evaluate organ-specific LDs, in skeletal muscle and liver, in response to a nutritional perturbation.
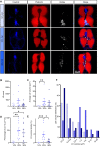
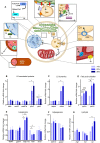
Methods: We developed a novel embedding and cryosectioning protocol allowing the conservation of LDs and organs in zebrafish larvae. To establish the quantitative measures, we used a three-arm parallel nutritional intervention design. Zebrafish larvae were fed a control diet containing 14% of nutritional fat or two high fat diets (HFDs) containing 25 and 36% of dietary fats. In muscle and liver, LDs were characterized using immunofluorescence confocal microscopy. In liver, intrahepatocellular lipids were discriminated from intrasinusoid lipids. To complete liver characteristics, fibrosis was identified with Masson's Trichrome staining. Finally, to confirm the conservation and effect of HFD, molecular players of fat metabolism were evaluated by RT-qPCR.
Results: The cryosections obtained after setting up the embedding and cryopreservation method were of high quality, preserving tissue morphology and allowing the visualization of ectopic lipids. Both HFDs were obesogenic, without modifying larvae survival or development. Neutral lipid content increased with time and augmented dietary fat. Intramuscular LD volume density increased and was explained by an increase in LDs size but not in numbers. Intrahepatocellular LD volume density increased and was explained by an increased number of LDs, not by their increased size. Sinusoid area and lipid content were both increased. Hepatic fibrosis appeared with both HFDs. We observed alterations in the expression of genes associated with LD coating proteins, LD dynamics, lipogenesis, lipolysis and fatty acid oxidation.
Conclusions: In this study, we propose a reproducible and fast method to image zebrafish larvae without losing LD quality and organ morphology. We demonstrate the impact of HFD on LD characteristics in liver and skeletal muscle accompanied by alterations of key players of fat metabolism. Our observations confirm the evolutionarily conserved mechanisms in lipid metabolism and reveal organ specific adaptations. The methodological advancements proposed in this work open the doors to study organelle adaptations in obesity and diabetes related research such as lipotoxicity, organelle contacts and specific lipid depositions.
Keywords: Fatty liver; Intrahepatic lipids; Intramyocellular lipids; Lipid droplets; Lipid metabolism; Obesity; Zebrafish.
Copyright © 2023 The Author(s). Published by Elsevier GmbH.. All rights reserved.
Conflict of interest statement
Conflict of Interest None declared.
Figures




Similar articles
-
Enhanced lipid oxidation and maintenance of muscle insulin sensitivity despite glucose intolerance in a diet-induced obesity mouse model.PLoS One. 2013 Aug 12;8(8):e71747. doi: 10.1371/journal.pone.0071747. eCollection 2013. PLoS One. 2013. PMID: 23951235 Free PMC article.
-
Lipid droplets are both highly oxidized and Plin2-covered in hepatocytes of diet-induced obese mice.Appl Physiol Nutr Metab. 2020 Dec;45(12):1368-1376. doi: 10.1139/apnm-2019-0966. Epub 2020 Jun 25. Appl Physiol Nutr Metab. 2020. PMID: 32585124
-
Dissociation of intramyocellular lipid storage and insulin resistance in trained athletes and type 2 diabetes patients; involvement of perilipin 5?J Physiol. 2018 Mar 1;596(5):857-868. doi: 10.1113/JP275182. Epub 2017 Nov 23. J Physiol. 2018. PMID: 29110300 Free PMC article.
-
Skeletal Muscle Lipid Droplets and the Athlete's Paradox.Cells. 2019 Mar 15;8(3):249. doi: 10.3390/cells8030249. Cells. 2019. PMID: 30875966 Free PMC article. Review.
-
The role of lipid droplets in metabolic disease in rodents and humans.J Clin Invest. 2011 Jun;121(6):2102-10. doi: 10.1172/JCI46069. Epub 2011 Jun 1. J Clin Invest. 2011. PMID: 21633178 Free PMC article. Review.
Cited by
-
Cluster Analysis in Diabetes Research: A Systematic Review Enhanced by a Cross-Sectional Study.J Clin Med. 2025 May 21;14(10):3588. doi: 10.3390/jcm14103588. J Clin Med. 2025. PMID: 40429583 Free PMC article. Review.
-
Citrus p-Synephrine Improves Energy Homeostasis by Regulating Amino Acid Metabolism in HFD-Induced Mice.Nutrients. 2024 Jan 12;16(2):248. doi: 10.3390/nu16020248. Nutrients. 2024. PMID: 38257140 Free PMC article.
-
Multi-tissue metabolomics reveal mtDNA- and diet-specific metabolite profiles in a mouse model of cardiometabolic disease.Redox Biol. 2025 Apr;81:103541. doi: 10.1016/j.redox.2025.103541. Epub 2025 Feb 14. Redox Biol. 2025. PMID: 39983345 Free PMC article.
-
Sustained feeding of a diet high in fat resulted in a decline in the liver's insulin-degrading enzyme levels in association with the induction of oxidative and endoplasmic reticulum stress in adult male rats: Evaluation of 4-phenylbutyric acid.Heliyon. 2024 Jun 10;10(12):e32804. doi: 10.1016/j.heliyon.2024.e32804. eCollection 2024 Jun 30. Heliyon. 2024. PMID: 38975085 Free PMC article.
-
Elocalcitol mitigates high-fat diet-induced microglial senescence via miR-146a modulation.Immun Ageing. 2024 Nov 22;21(1):82. doi: 10.1186/s12979-024-00485-6. Immun Ageing. 2024. PMID: 39578804 Free PMC article.
References
-
- Unger R.H., Orci L. Diseases of liporegulation: new perspective on obesity and related disorders. Faseb J. 2001;15(2):312–321. - PubMed
-
- Szendroedi J., Roden M. Ectopic lipids and organ function. Curr Opin Lipidol. 2009:50–56. - PubMed
-
- Schrauwen P., Westerterp K.R. The role of high-fat diets and physical activity in the regulation of body weight. Br J Nutr. 2000:417–427. CAB International. - PubMed
-
- Badin P.M., Langin D., Moro C. Dynamics of skeletal muscle lipid pools. TEM (Trends Endocrinol Metab) 2013;24(12):607–615. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

