Mitochondria-containing extracellular vesicles (EV) reduce mouse brain infarct sizes and EV/HSP27 protect ischemic brain endothelial cultures
- PMID: 36642252
- PMCID: PMC9974867
- DOI: 10.1016/j.jconrel.2023.01.025
Mitochondria-containing extracellular vesicles (EV) reduce mouse brain infarct sizes and EV/HSP27 protect ischemic brain endothelial cultures
Abstract
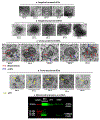
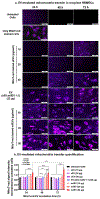
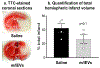
Ischemic stroke causes brain endothelial cell (BEC) death and damages tight junction integrity of the blood-brain barrier (BBB). We harnessed the innate mitochondrial load of BEC-derived extracellular vesicles (EVs) and utilized mixtures of EV/exogenous 27 kDa heat shock protein (HSP27) as a one-two punch strategy to increase BEC survival (via EV mitochondria) and preserve their tight junction integrity (via HSP27 effects). We demonstrated that the medium-to-large (m/lEV) but not small EVs (sEV) transferred their mitochondrial load, that subsequently colocalized with the mitochondrial network of the recipient primary human BECs. Recipient BECs treated with m/lEVs showed increased relative ATP levels and mitochondrial function. To determine if the m/lEV-meditated increase in recipient BEC ATP levels was associated with m/lEV mitochondria, we isolated m/lEVs from donor BECs pre-treated with oligomycin A (OGM, mitochondria electron transport complex V inhibitor), referred to as OGM-m/lEVs. BECs treated with naïve m/lEVs showed a significant increase in ATP levels compared to untreated OGD cells, OGM-m/lEVs treated BECs showed a loss of ATP levels suggesting that the m/lEV-mediated increase in ATP levels is likely a function of their innate mitochondrial load. In contrast, sEV-mediated ATP increases were not affected by inhibition of mitochondrial function in the donor BECs. Intravenously administered m/lEVs showed a reduction in brain infarct sizes compared to vehicle-injected mice in a mouse middle cerebral artery occlusion model of ischemic stroke. We formulated binary mixtures of human recombinant HSP27 protein with EVs: EV/HSP27 and ternary mixtures of HSP27 and EVs with a cationic polymer, poly (ethylene glycol)-b-poly (diethyltriamine): (PEG-DET/HSP27)/EV. (PEG-DET/HSP27)/EV and EV/HSP27 mixtures decreased the paracellular permeability of small and large molecular mass fluorescent tracers in oxygen glucose-deprived primary human BECs. This one-two punch approach to increase BEC metabolic function and tight junction integrity may be a promising strategy for BBB protection and prevention of long-term neurological dysfunction post-ischemic stroke.
Keywords: BBB protection; Extracellular vesicles; Heat shock protein; Ischemic stroke; Mitochondria; Paracellular permeability.
Copyright © 2023 Elsevier B.V. All rights reserved.
Figures







References
-
- He Z, Ning N, Zhou Q, Khoshnam SE, Farzaneh M. Mitochondria as a therapeutic target for ischemic stroke. Free Radical Biology and Medicine. 2020;146:45–58. - PubMed
-
- Bernardo-Castro S, Sousa JA, Brás A, Cecília C, Rodrigues B, Almendra L, et al. Pathophysiology of Blood–Brain Barrier Permeability Throughout the Different Stages of Ischemic Stroke and Its Implication on Hemorrhagic Transformation and Recovery. Frontiers in Neurology. 2020;11(1605). - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

