Membrane reshaping by protein condensates
- PMID: 36642341
- PMCID: PMC10208392
- DOI: 10.1016/j.bbamem.2023.184121
Membrane reshaping by protein condensates
Abstract
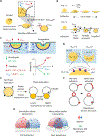
Proteins can organize into dynamic, functionally important assemblies on fluid membrane surfaces. Phase separation has emerged as an important mechanism for forming such protein assemblies on the membrane during cell signaling, endocytosis, and cytoskeleton regulation. Protein-protein phase separation thus adds novel fluid mosaics to the classical Singer and Nicolson model. Protein condensates formed in this process can modulate membrane morphologies. This is evident from recent reports of protein condensate-driven membrane reshaping in processes such as endocytosis, autophagosome formation, and protein storage vacuole morphogenesis in plants. Lateral phase separation (on the membrane surface) of peripheral curvature coupling proteins can modulate such membrane morphological transitions. Additionally, three-dimensional protein phase separation can result in droplets that through adhesion can affect membrane shape changes. How do these condensate-driven curvature generation mechanisms contrast with the classically recognized scaffolding and amphipathic helix insertion activities of specific membrane remodeling proteins? A salient feature of these condensate-driven membrane activities is that they depend upon both macroscopic features (such as interfacial energies of the condensate, membrane, and cytosol) as well as microscopic, molecular-level interactions (such as protein-lipid binding). This review highlights the current understanding of the mechanisms underlying curvature generation by protein condensates in various biological pathways.
Keywords: Condensates; Endocytosis; Membrane curvature; Phase separation.
Copyright © 2023 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Shin Y, Brangwynne CP, Liquid phase condensation in cell physiology and disease, Science 357(6357) (2017). - PubMed
-
- Agudo-Canalejo J, Schultz SW, Chino H, Migliano SM, Saito C, Koyama-Honda I, Stenmark H, Brech A, May AI, Mizushima N, Knorr RL, Wetting regulates autophagy of phase-separated compartments and the cytosol, Nature 591(7848) (2021) 142–146. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

