The foundation and architecture of precision medicine in neurology and psychiatry
- PMID: 36642626
- PMCID: PMC10720395
- DOI: 10.1016/j.tins.2022.12.004
The foundation and architecture of precision medicine in neurology and psychiatry
Abstract
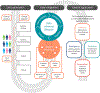
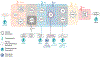
Neurological and psychiatric diseases have high degrees of genetic and pathophysiological heterogeneity, irrespective of clinical manifestations. Traditional medical paradigms have focused on late-stage syndromic aspects of these diseases, with little consideration of the underlying biology. Advances in disease modeling and methodological design have paved the way for the development of precision medicine (PM), an established concept in oncology with growing attention from other medical specialties. We propose a PM architecture for central nervous system diseases built on four converging pillars: multimodal biomarkers, systems medicine, digital health technologies, and data science. We discuss Alzheimer's disease (AD), an area of significant unmet medical need, as a case-in-point for the proposed framework. AD can be seen as one of the most advanced PM-oriented disease models and as a compelling catalyzer towards PM-oriented neuroscience drug development and advanced healthcare practice.
Keywords: Alzheimer’s disease; biomarkers; biomedical research; data science; digital health; systems medicine.
Copyright © 2022 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests H.H. is an employee of Eisai and serves as senior associate editor for the Journal Alzheimer’s & Dementia and has not received any fees or honoraria since May 2019. H.H. is inventor of 11 patents and has received no royalties for: In Vitro Multiparameter Determination Method for The Diagnosis and Early Diagnosis of Neurodegenerative Disorders patent no. 8916388; In Vitro Procedure for Diagnosis and Early Diagnosis of Neurodegenerative Diseases patent no. 8298784; Neurodegenerative Markers for Psychiatric Conditions publication no. 20120196300; In Vitro Multiparameter Determination Method for The Diagnosis and Early Diagnosis of Neurodegenerative Disorders publication no. 20100062463; In Vitro Method for The Diagnosis and Early Diagnosis of Neurodegenerative Disorders publication no. 20100035286; In Vitro Procedure for Diagnosis and Early Diagnosis of Neurodegenerative Diseases publication no. 20090263822; In Vitro Method for The Diagnosis of Neurodegenerative Diseases patent no. 7547553; CSF Diagnostic in Vitro Method for Diagnosis of Dementias and Neuroinflammatory Diseases publication no. 20080206797; In Vitro Method for The Diagnosis of Neurodegenerative Diseases publication no. 20080199966; Neurodegenerative Markers for Psychiatric Conditions publication no. 20080131921; Method for diagnosis of dementias and neuroinflammatory diseases based on an increased level of procalcitonin in cerebrospinal fluid: US patent no. 10921330. P.G. is an employee of Eisai Inc. J.C. has provided consultation to AB Science, Acadia, Alkahest, AlphaCognition, ALZPathFinder, Annovis, AriBio, Artery, Avanir, Biogen, Biosplice, Cassava, Cerevel, Clinilabs, Cortexyme, Diadem, EIP Pharma, Eisai, GatehouseBio, GemVax, Genentech, Green Valley, Grifols, Janssen, Karuna, Lexeo, Lilly, Lundbeck, LSP, Merck, NervGen, Novo Nordisk, Oligomerix, Otsuka, PharmacotrophiX, PRODEO, Prothena, ReMYND, Renew, Resverlogix, Roche, Signant Health, Suven, Unlearn AI, Vaxxinity, VigilNeuro pharmaceutical, assessment, and investment companies. N.T. has provided consultation to Eisai. P.M.T. has received a research grant from Biogen, Inc., for work unrelated to this manuscript, and has provided consultation to Kairos Venture Capital, Inc. Y.H. is an employee of Eisai Inc. M.C. is an employee of Eisai Inc. A.V. declares no competing financial interests related to the present article, and his contribution to this article reflects entirely and only his own academic expertise on the matter. A.V. was an employee of Eisai Inc. (Nov 2019–June 2021). A.V. does not receive any fees or honoraria since November 2019. Before November 2019 he had received lecture honoraria from Roche, MagQu LLC, and Servier.
Figures


References
-
- Kesselheim AS et al. (2015) Two decades of new drug development for central nervous system disorders. Nat. Rev. Drug Discov 14, 815–816 - PubMed
Publication types
MeSH terms
Grants and funding
- R01 AG058854/AG/NIA NIH HHS/United States
- P41 EB015922/EB/NIBIB NIH HHS/United States
- P20 AG068053/AG/NIA NIH HHS/United States
- R35 AG071476/AG/NIA NIH HHS/United States
- U01 AG068057/AG/NIA NIH HHS/United States
- U01 NS093334/NS/NINDS NIH HHS/United States
- R01 MH116147/MH/NIMH NIH HHS/United States
- RF1 AG057892/AG/NIA NIH HHS/United States
- R01 AG053798/AG/NIA NIH HHS/United States
- R01 NS107513/NS/NINDS NIH HHS/United States
- RF1 MH123163/MH/NIMH NIH HHS/United States
- P20 GM109025/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

