Obstructive sleep apnea is related to alterations in fecal microbiome and impaired intestinal barrier function
- PMID: 36642764
- PMCID: PMC9841009
- DOI: 10.1038/s41598-023-27784-0
Obstructive sleep apnea is related to alterations in fecal microbiome and impaired intestinal barrier function
Abstract
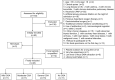
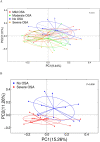
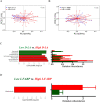
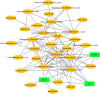
Obstructive Sleep Apnea (OSA) is related to repeated upper airway collapse, intermittent hypoxia, and intestinal barrier dysfunction. The resulting damage to the intestinal barrier may affect or be affected by the intestinal microbiota. A prospective case-control was used, including 48 subjects from Sleep Medicine Center of Nanfang Hospital. Sleep apnea was diagnosed by overnight polysomnography. Fecal samples and blood samples were collected from subjects to detect fecal microbiome composition (by 16S rDNA gene amplification and sequencing) and intestinal barrier biomarkers-intestinal fatty acid-binding protein (I-FABP) and D-lactic acid (D-LA) (by ELISA and colorimetry, respectively). Plasma D-LA and I-FABP were significantly elevated in patients with OSA. The severity of OSA was related to differences in the structure and composition of the fecal microbiome. Enriched Fusobacterium, Megamonas, Lachnospiraceae_UCG_006, and reduced Anaerostipes was found in patients with severe OSA. Enriched Ruminococcus_2, Lachnoclostridium, Lachnospiraceae_UCG_006, and Alloprevotella was found in patients with high intestinal barrier biomarkers. Lachnoclostridium and Lachnospiraceae_UCG_006 were the common dominant bacteria of OSA and intestinal barrier damage. Fusobacterium and Peptoclostridium was independently associated with apnea-hypopnea index (AHI). The dominant genera of severe OSA were also related to glucose, lipid, neutrophils, monocytes and BMI. Network analysis identified links between the fecal microbiome, intestinal barrier biomarkers, and AHI. The study confirms that changes in the intestinal microbiota are associated with intestinal barrier biomarkers among patients in OSA. These changes may play a pathophysiological role in the systemic inflammation and metabolic comorbidities associated with OSA, leading to multi-organ morbidity of OSA.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Impaired intestinal barrier in patients with obstructive sleep apnea.Sleep Breath. 2021 Jun;25(2):749-756. doi: 10.1007/s11325-020-02178-y. Epub 2020 Aug 26. Sleep Breath. 2021. PMID: 32845474
-
Analysis of the characteristics of intestinal microbiota in patients with different severity of obstructive sleep apnea.Sci Rep. 2024 Sep 16;14(1):21552. doi: 10.1038/s41598-024-72230-4. Sci Rep. 2024. PMID: 39285240 Free PMC article.
-
Sleep apnea is associated with the increase of certain genera of Ruminococcaceae and Lachnospiraceae in the gut microbiome of hypertensive patients.Expert Rev Respir Med. 2022 Nov-Dec;16(11-12):1247-1256. doi: 10.1080/17476348.2022.2147509. Epub 2022 Nov 20. Expert Rev Respir Med. 2022. PMID: 36369876
-
Metabolomics and microbiome profiling as biomarkers in obstructive sleep apnoea: a comprehensive review.Eur Respir Rev. 2021 May 11;30(160):200220. doi: 10.1183/16000617.0220-2020. Print 2021 Jun 30. Eur Respir Rev. 2021. PMID: 33980666 Free PMC article. Review.
-
Exploring the potential relationships among obstructive sleep apnea, erectile dysfunction, and gut microbiota: a narrative review.Sex Med Rev. 2023 Dec 23;12(1):76-86. doi: 10.1093/sxmrev/qead026. Sex Med Rev. 2023. PMID: 37385976 Review.
Cited by
-
The Burden of Comorbidities in Obstructive Sleep Apnea and the Pathophysiologic Mechanisms and Effects of CPAP.Clocks Sleep. 2023 Jun 19;5(2):333-349. doi: 10.3390/clockssleep5020025. Clocks Sleep. 2023. PMID: 37366660 Free PMC article. Review.
-
Randomized controlled trial comparing the impacts of Saccharomyces boulardii and Lactobacillus rhamnosus OF44 on intestinal flora in cerebral palsy rats: insights into inflammation biomarkers and depression-like behaviors.Transl Pediatr. 2024 Jan 29;13(1):72-90. doi: 10.21037/tp-23-566. Epub 2024 Jan 24. Transl Pediatr. 2024. PMID: 38323178 Free PMC article.
-
Back to Roots: Dysbiosis, Obesity, Metabolic Syndrome, Type 2 Diabetes Mellitus, and Obstructive Sleep Apnea-Is There an Objective Connection? A Narrative Review.Nutrients. 2024 Nov 26;16(23):4057. doi: 10.3390/nu16234057. Nutrients. 2024. PMID: 39683451 Free PMC article. Review.
-
Impact of Obstructive Sleep Apnea and Triglyceride Glucose Index on Cardiovascular Events in Acute Coronary Syndrome Patients: A Post-Hoc Analysis of the OSA-ACS Study.Rev Cardiovasc Med. 2025 May 21;26(5):36205. doi: 10.31083/RCM36205. eCollection 2025 May. Rev Cardiovasc Med. 2025. PMID: 40475723 Free PMC article.
-
OSA Is Associated With the Human Gut Microbiota Composition and Functional Potential in the Population-Based Swedish CardioPulmonary bioImage Study.Chest. 2023 Aug;164(2):503-516. doi: 10.1016/j.chest.2023.03.010. Epub 2023 Mar 15. Chest. 2023. PMID: 36925044 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

