Catanionic Vesicles as a Facile Scaffold to Display Natural N-Glycan Ligands for Probing Multivalent Carbohydrate-Lectin Interactions
- PMID: 36642983
- PMCID: PMC10349922
- DOI: 10.1021/acs.bioconjchem.2c00560
Catanionic Vesicles as a Facile Scaffold to Display Natural N-Glycan Ligands for Probing Multivalent Carbohydrate-Lectin Interactions
Abstract
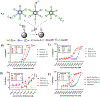
Multivalent interactions are a key characteristic of protein-carbohydrate recognition. Phospholipid-based liposomes have been explored as a popular platform for multivalent presentation of glycans, but this platform has been plagued by the instability of typical liposomal formulations in biological media. We report here the exploitation of catanionic vesicles as a stable lipid-based nanoparticle scaffold for displaying large natural N-glycans as multivalent ligands. Hydrophobic insertion of lipidated N-glycans into the catanionic vesicle bilayer was optimized to allow for high-density display of structurally diverse N-glycans on the outer membrane leaflet. In an enzyme-linked competitive lectin-binding assay, the N-glycan-coated vesicles demonstrated a clear clustering glycoside effect, with significantly enhanced affinity for the corresponding lectins including Sambucus nigra agglutinin (SNA), concanavalin A (ConA), and human galectin-3, in comparison with their respective natural N-glycan ligands. Our results showed that relatively low density of high-mannose and sialylated complex type N-glycans gave the maximal clustering effect for binding to ConA and SNA, respectively, while relatively high-density display of the asialylated complex type N-glycan provided maximal clustering effects for binding to human galectin 3. Moreover, we also observed a macromolecular crowding effect on the binding of ConA to high-mannose N-glycans when catanionic vesicles bearing mixed high-mannose and complex-type N-glycans were used. The N-glycan-coated catanionic vesicles are stable and easy to formulate with varied density of ligands, which could serve as a feasible vehicle for drug delivery and as potent inhibitors for intervening protein-carbohydrate interactions implicated in disease.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



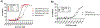
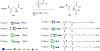
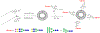
Similar articles
-
Carbohydrate-functionalized surfactant vesicles for controlling the density of glycan arrays.Talanta. 2012 Mar 15;91:134-9. doi: 10.1016/j.talanta.2012.01.036. Epub 2012 Jan 24. Talanta. 2012. PMID: 22365692
-
Carbohydrate modified catanionic vesicles: probing multivalent binding at the bilayer interface.J Am Chem Soc. 2009 Apr 22;131(15):5471-7. doi: 10.1021/ja8076439. J Am Chem Soc. 2009. PMID: 19323555
-
Glycan-Gold Nanoparticles as Multifunctional Probes for Multivalent Lectin-Carbohydrate Binding: Implications for Blocking Virus Infection and Nanoparticle Assembly.J Am Chem Soc. 2020 Oct 21;142(42):18022-18034. doi: 10.1021/jacs.0c06793. Epub 2020 Sep 29. J Am Chem Soc. 2020. PMID: 32935985
-
Legume Lectins with Different Specificities as Potential Glycan Probes for Pathogenic Enveloped Viruses.Cells. 2022 Jan 20;11(3):339. doi: 10.3390/cells11030339. Cells. 2022. PMID: 35159151 Free PMC article. Review.
-
Plant lectins and their usage in preparing targeted nanovaccines for cancer immunotherapy.Semin Cancer Biol. 2022 May;80:87-106. doi: 10.1016/j.semcancer.2020.02.005. Epub 2020 Feb 14. Semin Cancer Biol. 2022. PMID: 32068087 Review.
Cited by
-
Simplified Synthesis of Poly(ethyleneimine)-Modified Silica Particles and Their Application in Oligosaccharide Isolation Methods.Int J Mol Sci. 2024 Aug 30;25(17):9465. doi: 10.3390/ijms25179465. Int J Mol Sci. 2024. PMID: 39273411 Free PMC article.
-
Soybean Lectin Cross-Links Membranes by Binding Sulfatide in a Curvature-Dependent Manner.J Agric Food Chem. 2025 Jun 4;73(22):14020-14031. doi: 10.1021/acs.jafc.5c04336. Epub 2025 May 24. J Agric Food Chem. 2025. PMID: 40411535 Free PMC article.
References
-
- Cerliani JP; Blidner AG; Toscano MA; Croci DO; Rabinovich GA Translating the ‘Sugar Code’ into Immune and Vascular Signaling Programs. Trends Biochem. Sci 2017, 42, 255–273. - PubMed
-
- Lee YC; Lee RT Carbohydrate-protein interactions: Basis of glycobiology. Acc. Chem. Res 1995, 28, 321–327.
-
- Lundquist JJ; Toone EJ The cluster glycoside effect. Chem. Rev 2002, 102, 555–578. - PubMed
-
- Mammen M; Choi SK; Whitesides GM Polyvalent interactions in biological systems: Implications for design and use of multivalent ligands and inhibitors. Angew. Chem., Int. Ed 1998, 37, 2754–2794. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

