Circ_0138960 knockdown alleviates lipopolysaccharide-induced inflammatory response and injury in human dental pulp cells by targeting miR-545-5p/MYD88 axis in pulpitis
- PMID: 36643232
- PMCID: PMC9831794
- DOI: 10.1016/j.jds.2022.06.012
Circ_0138960 knockdown alleviates lipopolysaccharide-induced inflammatory response and injury in human dental pulp cells by targeting miR-545-5p/MYD88 axis in pulpitis
Abstract
Background/purpose: Circular RNAs (circRNAs) have been shown to play important regulatory roles in many human diseases, yet their functions in pulpitis remain to be clarified. This study was designed to investigate the function of circ_0138960 in pulpitis progression and its underlying mechanism.
Material and methods: Cell viability and proliferation were analyzed by 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay and 5-Ethynyl-2'-deoxyuridine (EdU) assay. Flow cytometry and enzyme-linked immunosorbent assay (ELISA) were conducted to analyze cell apoptosis rate and the release of inflammatory cytokines. The activity of superoxide dismutase (SOD) was analyzed using a SOD assay kit. Dual-luciferase reporter and RNA-pull down assays were performed to verify the interaction between microRNA-545-5p (miR-545-5p) and circ_0138960 or myeloid differentiation primary response gene 88 (MYD88).
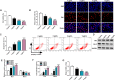
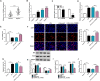
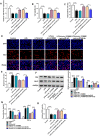
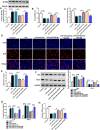
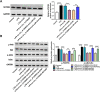
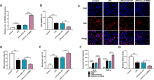
Results: Lipopolysaccharide (LPS) treatment restrained the proliferation and promoted the apoptosis, inflammation, and oxidative stress of human dental pulp cells (hDPCs). LPS treatment dose-dependently up-regulated circ_0138960 expression in hDPCs. Circ_0138960 knockdown overturned LPS-induced inflammation and injury in hDPCs. Circ_0138960 could act as a molecular sponge for miR-545-5p, and circ_0138960 knockdown protected hDPCs from LPS-induced effects by up-regulating miR-545-5p. miR-545-5p directly interacted with the 3' untranslated region (3'UTR) of MYD88, and MYD88 overexpression reversed miR-545-5p-mediated effects in LPS-treated hDPCs. Circ_0138960 positively regulated MYD88 expression by sponging miR-545-5p in hDPCs. LPS could activate nuclear factor kappa-B (NF-κB) signaling by targeting circ_0138960/miR-545-5p/MYD88 axis in hDPCs.
Conclusion: Circ_0138960 knockdown attenuated LPS-induced inflammatory response and injury in hDPCs by targeting the miR-545-5p/MYD88/NF-κB axis.
Keywords: MYD88; NF-κB; Pulpitis; circ_0138960; miR-545-5p.
© 2022 Association for Dental Sciences of the Republic of China. Publishing services by Elsevier B.V.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


Similar articles
-
NUTM2A-AS1 silencing alleviates LPS-induced apoptosis and inflammation in dental pulp cells through targeting let-7c-5p/HMGB1 axis.Int Immunopharmacol. 2021 Jul;96:107497. doi: 10.1016/j.intimp.2021.107497. Epub 2021 Apr 5. Int Immunopharmacol. 2021. Retraction in: Int Immunopharmacol. 2025 Mar 26;150:114314. doi: 10.1016/j.intimp.2025.114314. PMID: 33831808 Retracted.
-
Circ-BICC1 Knockdown Alleviates Lipopolysaccharide (LPS)-Induced WI-38 Cell Injury Through miR-338-3p/MYD88 Axis.Biochem Genet. 2023 Feb;61(1):170-186. doi: 10.1007/s10528-022-10242-3. Epub 2022 Jul 9. Biochem Genet. 2023. PMID: 35809112
-
Circ_0043947 contributes to interleukin 1β-induced injury in chondrocytes by sponging miR-671-5p to up-regulate RTN3 expression in osteoarthritis pathology.J Orthop Surg Res. 2022 Mar 24;17(1):177. doi: 10.1186/s13018-022-02970-4. J Orthop Surg Res. 2022. PMID: 35331286 Free PMC article.
-
KNOCKDOWN OF CIRC_0114428 ALLEVIATES LPS-INDUCED HK2 CELL APOPTOSIS AND INFLAMMATION INJURY VIA TARGETING MIR-215-5P/TRAF6/NF-ΚB AXIS IN SEPTIC ACUTE KIDNEY INJURY.Shock. 2024 Apr 1;61(4):620-629. doi: 10.1097/SHK.0000000000002245. Epub 2023 Nov 15. Shock. 2024. PMID: 38010029
-
Circ_0000396 suppresses the proliferation and inflammation of rheumatoid arthritis synovial fibroblasts by targeting miR-574-5p/RSPO1 axis.J Orthop Surg Res. 2023 Sep 22;18(1):718. doi: 10.1186/s13018-023-04117-5. J Orthop Surg Res. 2023. PMID: 37737195 Free PMC article.
Cited by
-
Potential of Dental Pulp Stem Cell Exosomes: Unveiling miRNA-Driven Regenerative Mechanisms.Int Dent J. 2025 Apr;75(2):415-425. doi: 10.1016/j.identj.2024.08.019. Epub 2024 Oct 5. Int Dent J. 2025. PMID: 39368923 Free PMC article. Review.
-
Dysregulation of MicroRNA-455-5p Contributes to Pulpitis Pathogenesis Through Modulating MYD88.Int Dent J. 2025 Aug 4;75(5):100933. doi: 10.1016/j.identj.2025.100933. Online ahead of print. Int Dent J. 2025. PMID: 40763691 Free PMC article.
-
Current Insights into the Roles of LncRNAs and CircRNAs in Pulpitis: A Narrative Review.Int J Mol Sci. 2024 Dec 19;25(24):13603. doi: 10.3390/ijms252413603. Int J Mol Sci. 2024. PMID: 39769365 Free PMC article. Review.
-
circ_0002456/FUS interaction inhibits NF-κB signaling to attenuate DNA damage and inflammatory responses in hDPSCs.Stem Cell Res Ther. 2025 Jun 2;16(1):276. doi: 10.1186/s13287-025-04391-6. Stem Cell Res Ther. 2025. PMID: 40457486 Free PMC article.
-
Mechanistic insights into dental stem cells-derived exosomes in regenerative endodontics.Int Endod J. 2025 Sep;58(9):1384-1407. doi: 10.1111/iej.14269. Epub 2025 Jun 11. Int Endod J. 2025. PMID: 40497413 Free PMC article. Review.
References
-
- Kang K.J., Ko S.Y., Ryu C.J., Jang Y.J. A monoclonal antibody recognizes undifferentiation-specific carbohydrate moieties expressed on cell surface of the human dental pulp cells. Stem Cell Res. 2017;21:85–93. - PubMed
-
- Yonehiro J., Yamashita A., Yoshida Y., et al. Establishment of an ex vivo pulpitis model by co-culturing immortalized dental pulp cells and macrophages. Int Endod J. 2012;45:1103–1108. - PubMed
-
- Morsczeck C., Reichert T.E. Dental stem cells in tooth regeneration and repair in the future. Expet Opin Biol Ther. 2018;18:187–196. - PubMed
-
- Nuti N., Corallo C., Chan B.M., Ferrari M., Gerami-Naini B. Multipotent differentiation of human dental pulp stem cells: a literature review. Stem Cell Rev Rep. 2016;12:511–523. - PubMed
LinkOut - more resources
Full Text Sources

