Anti-apoptosis and anti-inflammation activity of circ_0097010 downregulation in lipopolysaccharide-stimulated periodontal ligament cells by miR-769-5p/Krüppel like factor 6 axis
- PMID: 36643256
- PMCID: PMC9831795
- DOI: 10.1016/j.jds.2022.04.024
Anti-apoptosis and anti-inflammation activity of circ_0097010 downregulation in lipopolysaccharide-stimulated periodontal ligament cells by miR-769-5p/Krüppel like factor 6 axis
Abstract
Background/purpose: Periodontitis is a prevalent infectious inflammatory disease. Growing evidence has revealed important roles for circular RNAs (circRNAs) and circRNA sponge activity in periodontitis. Here, we elucidated the precise part of circ_0097010 in periodontitis pathogenesis.
Materials and methods: Human periodontal ligament cells (hPDLCs) were exposed to lipopolysaccharide (LPS). Cell viability, proliferation and apoptosis were evaluated by CCK-8 assay, EdU incorporation assay and flow cytometry, respectively. Circ_0097010, microRNA (miR)-769-5p and Krüppel like factor 6 (KLF6) were quantified by qRT-PCR and Western blot. Interleukin 6 (IL-6) level, tumor necrosis factor-α (TNF-α) secretion, superoxide dismutase (SOD) activity and malondialdehyde (MDA) level were detected by enzyme-linked immunosorbent assay (ELISA). Dual-luciferase reporter, RNA immunoprecipitation (RIP) and RNA pull-down assays were used to confirm the direct relationship between miR-769-5p and circ_0097010 or KLF6.
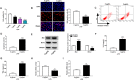
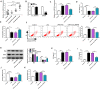
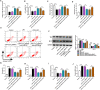
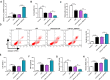
Results: Our data showed that LPS repressed cell proliferation and induced cell apoptosis and inflammation in hPDLCs. Circ_0097010 was upregulated in periodontitis samples and LPS-exposed hPDLCs. Downregulation of circ_0097010 exerted anti-apoptosis and anti-inflammation functions in LPS-exposed hPDLCs. Mechanistically, circ_0097010 acted as a miR-769-5p sponge, and reduced abundance of miR-769-5p reversed the anti-apoptosis and anti-inflammation effects of circ_0097010 suppression. KLF6 was a direct miR-769-5p target, and miR-769-5p-mediated inhibition of KLF6 possessed anti-apoptosis and anti-inflammation functions in LPS-induced hPDLCs. Moreover, circ_0097010 controlled KLF6 expression by miR-769-5p.
Conclusion: These data identify circ_0097010 as a key regulator of LPS-induced inflammation and apoptosis in hPDLCs and highlight a novel mechanism of circ_0097010 regulation through miR-769-5p/KLF6 axis.
Keywords: Circ_0097010; Inflammation; Krüppel like factor 6 (KLF6); MiR-769-5p; Periodontitis.
© 2022 Association for Dental Sciences of the Republic of China. Publishing services by Elsevier B.V.
Conflict of interest statement
The authors have no conflicts of interest relevant to this article.
Figures




References
-
- Bartold P.M. Lifestyle and periodontitis: the emergence of personalized periodontics. Periodontol 2000. 2018;78:7–11. - PubMed
-
- Kristensen L.S., Andersen M.S., Stagsted L.V.W., Ebbesen K.K., Hansen T.B., Kjems J. The biogenesis, biology and characterization of circular RNAs. Nat Rev Genet. 2019;20:675–691. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

