Combined application of plant growth-promoting bacteria and iron oxide nanoparticles ameliorates the toxic effects of arsenic in Ajwain (Trachyspermum ammi L.)
- PMID: 36643291
- PMCID: PMC9832315
- DOI: 10.3389/fpls.2022.1098755
Combined application of plant growth-promoting bacteria and iron oxide nanoparticles ameliorates the toxic effects of arsenic in Ajwain (Trachyspermum ammi L.)
Abstract
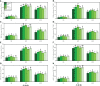
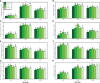
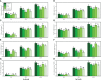
Soil contamination with toxic heavy metals [such as arsenic (As)] is becoming a serious global problem because of the rapid development of the social economy. Although plant growth-promoting bacteria (PGPB) and nanoparticles (NPs) are the major protectants to alleviate metal toxicity, the study of these chemicals in combination to ameliorate the toxic effects of As is limited. Therefore, the present study was conducted to investigate the combined effects of different levels of Providencia vermicola (5 ppm and 10 ppm) and iron oxide nanoparticles (FeO-NPs) (50 mg/l-1 and 100 mg/l-1) on plant growth and biomass, photosynthetic pigments, gas exchange attributes, oxidative stress and response of antioxidant compounds (enzymatic and non-enzymatic), and their specific gene expression, sugars, nutritional status of the plant, organic acid exudation pattern As accumulation from the different parts of the plants, and electron microscopy under the soil, which was spiked with different levels of As [0 μM (i.e., no As), 50 μM, and 100 μM] in Ajwain (Trachyspermum ammi L.) seedlings. Results from the present study showed that the increasing levels of As in the soil significantly (p< 0.05) decreased plant growth and biomass, photosynthetic pigments, gas exchange attributes, sugars, and nutritional contents from the roots and shoots of the plants, and destroyed the ultra-structure of membrane-bound organelles. In contrast, increasing levels of As in the soil significantly (p< 0.05) increased oxidative stress indicators in term of malondialdehyde, hydrogen peroxide, and electrolyte leakage, and also increased organic acid exudation patter in the roots of T. ammi seedlings. The negative impact of As toxicity can overcome the application of PGPB (P. vermicola) and FeO-NPs, which ultimately increased plant growth and biomass by capturing the reactive oxygen species, and decreased oxidative stress in T. ammi seedlings by decreasing the As contents in the roots and shoots of the plants. Our results also showed that the FeO-NPs were more sever and showed better results when we compared with PGPB (P. vermicola) under the same treatment of As in the soil. Research findings, therefore, suggest that the combined application of P. vermicola and FeO-NPs can ameliorate As toxicity in T. ammi seedlings, resulting in improved plant growth and composition under metal stress, as depicted by balanced exudation of organic acids.
Keywords: Providencia vermicola; electron microanalysis; heavy metal; herbaceous crop; nano-technology.
Copyright © 2022 Sun, Ma, Ma, Li, Zhu and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







References
-
- Afzal J., Wang X., Saleem M.-H., Sun X., Hussain S., Khan I., et al. (2021). Application of ferrous sulfate alleviates negative impact of cadmium in rice (Oryza sativa l.). BIOCELL 45, 1631–1649. doi: 10.32604/biocell.2021.014934 - DOI
-
- Ahad F., Fatima A., Hakim S. T., Nadeem S. G. (2014). Synergistic antibacterial activity of trachyspermum ammi (Ajwain) and metal salts against pathogenic bacteria. RADS. J. Biol. Res. Appl. Sci. 5, 29–35.
LinkOut - more resources
Full Text Sources
Research Materials

