S-Nitrosoglutathione Reduces the Density of Staphylococcus aureus Biofilms Established on Human Airway Epithelial Cells
- PMID: 36643497
- PMCID: PMC9835527
- DOI: 10.1021/acsomega.2c06212
S-Nitrosoglutathione Reduces the Density of Staphylococcus aureus Biofilms Established on Human Airway Epithelial Cells
Abstract
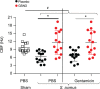
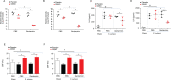
Patients with chronic rhinosinusitis (CRS) often show persistent colonization by bacteria in the form of biofilms which are resistant to antibiotic treatment. One of the most commonly isolated bacteria in CRS is Staphylococcus aureus (S. aureus). Nitric oxide (NO) is a potent antimicrobial agent and disperses biofilms efficiently. We hypothesized that S-nitrosoglutathione (GSNO), an endogenous NO carrier/donor, synergizes with gentamicin to disperse and reduce the bacterial biofilm density. We prepared GSNO formulations which are stable up to 12 months at room temperature and show the maximum amount of NO release within 1 h. We examined the effects of this GSNO formulation on the S. aureus biofilm established on the apical surface of the mucociliary-differentiated airway epithelial cell cultures regenerated from airway basal (stem) cells from cystic fibrosis (CF) and CRS patients. We demonstrate that for CF cells, which are defective in producing NO, treatment with GSNO at 100 μM increased the NO levels on the apical surface and reduced the biofilm bacterial density by 2 log units without stimulating pro-inflammatory effects or inducing epithelial cell death. In combination with gentamicin, GSNO further enhanced the killing of biofilm bacteria. Compared to placebo, GSNO significantly increased the ciliary beat frequency (CBF) in both infected and uninfected CF cell cultures. The combination of GSNO and gentamicin also reduced the bacterial density of biofilms grown on sinonasal epithelial cells from CRS patients and improved the CBF. These findings demonstrate that GSNO in combination with gentamicin may effectively reduce the density of biofilm bacteria in CRS patients. GSNO treatment may also enhance the mucociliary clearance by improving the CBF.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare the following competing financial interest(s): AW and GM are employees of the NOTA Labs MEM serves as Chief Technology Officer for NOTA Labs MZ, MBH, and US are scientific officers for NOTA Labs
Figures





Similar articles
-
Staphylococcus aureus Biofilm Growth on Cystic Fibrosis Airway Epithelial Cells Is Enhanced during Respiratory Syncytial Virus Coinfection.mSphere. 2018 Aug 15;3(4):e00341-18. doi: 10.1128/mSphere.00341-18. mSphere. 2018. PMID: 30111629 Free PMC article.
-
Acute effects of aerosolized S-nitrosoglutathione in cystic fibrosis.Am J Respir Crit Care Med. 2002 Apr 1;165(7):922-6. doi: 10.1164/ajrccm.165.7.2105032. Am J Respir Crit Care Med. 2002. PMID: 11934715 Clinical Trial.
-
Activation of chloride transport in CF airway epithelial cell lines and primary CF nasal epithelial cells by S-nitrosoglutathione.Respir Res. 2006 Oct 5;7(1):124. doi: 10.1186/1465-9921-7-124. Respir Res. 2006. PMID: 17022806 Free PMC article.
-
Roles and current applications of S-nitrosoglutathione in anti-infective biomaterials.Mater Today Bio. 2022 Sep 6;16:100419. doi: 10.1016/j.mtbio.2022.100419. eCollection 2022 Dec. Mater Today Bio. 2022. PMID: 36105674 Free PMC article. Review.
-
The mechanisms of biofilm antibiotic resistance in chronic rhinosinusitis: A review.Medicine (Baltimore). 2022 Dec 9;101(49):e32168. doi: 10.1097/MD.0000000000032168. Medicine (Baltimore). 2022. PMID: 36626427 Free PMC article. Review.
Cited by
-
Recreating chronic respiratory infections in vitro using physiologically relevant models.Eur Respir Rev. 2024 Aug 14;33(173):240062. doi: 10.1183/16000617.0062-2024. Print 2024 Jul. Eur Respir Rev. 2024. PMID: 39142711 Free PMC article. Review.
References
-
- Pleis J. R.; Ward B. W.; Lucas J. W. Summary health statistics for U.S. adults: National Health Interview Survey, 2009. Vital Health Statistics 2010, 249, 1–207. - PubMed
-
- Schiller J. S.; Lucas J. W.; Ward B. W.; Peregoy J. A. Summary health statistics for U.S. adults: National Health Interview Survey, 2010. Vital Health Statistics 2012, 252, 1–207. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

