Ni-Catalyzed Regioselective Reductive 1,3-Dialkenylation of Alkenes
- PMID: 36643521
- PMCID: PMC9835523
- DOI: 10.1021/acsomega.2c06417
Ni-Catalyzed Regioselective Reductive 1,3-Dialkenylation of Alkenes
Abstract
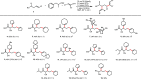
Dicarbofunctionalization is an important efficient synthetic technique for adding two chemical moieties across an alkene. Here, a novel method of reductive dicarbofunctionalization has been developed using a single alkenyl triflate as the electrophile, combined with an unactivated alkene. The reaction does not require an external auxiliary and proceeds with complete regioselectivity.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Recent Advances in First-Row Transition Metal-Catalyzed Reductive Coupling Reactions for π-Bond Functionalization and C-Glycosylation.Acc Chem Res. 2023 Nov 21;56(22):3292-3312. doi: 10.1021/acs.accounts.3c00531. Epub 2023 Nov 2. Acc Chem Res. 2023. PMID: 37917928
-
Transition Metal (Ni, Cu, Pd)-Catalyzed Alkene Dicarbofunctionalization Reactions.Acc Chem Res. 2021 Sep 7;54(17):3415-3437. doi: 10.1021/acs.accounts.1c00329. Epub 2021 Aug 12. Acc Chem Res. 2021. PMID: 34383469 Free PMC article.
-
Nickel-Catalyzed 8-Aminoquinoline Directed Reductive Dialkylcyclization/Homodialkylation of Unactivated Alkenes.Org Lett. 2023 Nov 3;25(43):7800-7804. doi: 10.1021/acs.orglett.3c02955. Epub 2023 Oct 24. Org Lett. 2023. PMID: 37874767
-
Enantioselective Dicarbofunctionalization of Unactivated Alkenes by Palladium-Catalyzed Tandem Heck/Suzuki Coupling Reaction.Angew Chem Int Ed Engl. 2019 Oct 7;58(41):14653-14659. doi: 10.1002/anie.201907840. Epub 2019 Sep 5. Angew Chem Int Ed Engl. 2019. PMID: 31420928 Review.
-
Three-component 1,2-dicarbofunctionalization of alkenes involving alkyl radicals.Chem Commun (Camb). 2022 Jan 18;58(6):730-746. doi: 10.1039/d1cc05730h. Chem Commun (Camb). 2022. PMID: 34931629 Review.
Cited by
-
Improving reproducibility through condition-based sensitivity assessments: application, advancement and prospect.Chem Sci. 2024 Aug 30;15(36):14548-55. doi: 10.1039/d4sc03017f. Online ahead of print. Chem Sci. 2024. PMID: 39263664 Free PMC article. Review.
-
Pd-Catalyzed 1,3-Alkenylarylation of Skipped Diene via Metal Migration.ACS Omega. 2023 May 25;8(22):19912-19916. doi: 10.1021/acsomega.3c01839. eCollection 2023 Jun 6. ACS Omega. 2023. PMID: 37305246 Free PMC article.
-
Cross-Electrophile Coupling: Principles, Methods, and Applications in Synthesis.Chem Rev. 2024 Dec 11;124(23):13397-13569. doi: 10.1021/acs.chemrev.4c00524. Epub 2024 Nov 26. Chem Rev. 2024. PMID: 39591522 Free PMC article. Review.
References
-
- Dhungana R. K.; Shekhar K. C.; Basnet P.; Giri R. Transition Metal-Catalyzed Dicarbofunctionalization of Unactivated Olefins. Chem. Rec. 2018, 18, 1314–1340. 10.1002/tcr.201700098. - DOI - PubMed
- Wickham L. M.; Giri R. Transition Metal (Ni, Cu, Pd)-Catalyzed Alkene Dicarbofunctionalization Reactions. Acc. Chem. Res. 2021, 54, 3415–3437. 10.1021/acs.accounts.1c00329. - DOI - PMC - PubMed
- Qi X. X.; Diao T. N. Nickel-Catalyzed Dicarbofunctionalization of Alkenes. ACS Catal. 2020, 10, 8542–8556. 10.1021/acscatal.0c02115. - DOI - PMC - PubMed
- Montgomery J. Nickel-catalyzed cyclizations, couplings, and cycloadditions involving three reactive components. Acc. Chem. Res. 2000, 33, 467–473. 10.1021/ar990095d. - DOI - PubMed
- Badir S. O.; Molander G. A. Developments in Photoredox/Nickel Dual-Catalyzed 1,2-Difunctionalizations. Chem 2020, 6, 1327–1339. 10.1016/j.chempr.2020.05.013. - DOI - PMC - PubMed
-
A preprint of the current work was deposited on an archive. See:
- Wickham L. M.; Dhungana R. K.; Giri R. Ni-catalyzed Regioselective Reductive 1,3-Dialkenylation of Alkenes. ChemRxiv 2022, 10.26434/chemrxiv-2022-8jrzc. - DOI - PMC - PubMed
-
- Derosa J.; Apolinar O.; Kang T.; Tran V. T.; Engle K. M. Recent developments in nickel-catalyzed intermolecular dicarbofunctionalization of alkenes. Chem. Sci. 2020, 11, 4287–4296. 10.1039/C9SC06006E. - DOI - PMC - PubMed
- Zhang J. S.; Liu L.; Chen T.; Han L. B. Transition-Metal-Catalyzed Three-Component Difunctionalizations of Alkenes. Chem. – Asian J. 2018, 13, 2277–2291. 10.1002/asia.201800647. - DOI - PubMed
-
- Xia T.; Xi Y.; Ding H.; Zhang Y.; Fang K.; Wu X.; Qu J.; Chen Y. Palladium(II)-catalyzed enantioselective intermolecular oxidative diarylation of internal enamides. Chem. Commun. 2022, 58, 9282–9285. 10.1039/D2CC03202C. - DOI - PubMed
- Ma Y. Y.; Zhang D. C.; Yan Z. Y.; Wang M. F.; Bian C. L.; Gao X. L.; Bunel E. E.; Lei A. W. Iron-Catalyzed Oxidative C-H/C-H Cross-Coupling between Electron-Rich Arenes and Alkenes. Org. Lett. 2015, 17, 2174–2177. 10.1021/acs.orglett.5b00775. - DOI - PubMed
- Werner E. W.; Sigman M. S. A Highly Selective and General Palladium Catalyst for the Oxidative Heck Reaction of Electronically Nonbiased Olefins. J. Am. Chem. Soc. 2010, 132, 13981–13983. 10.1021/ja1060998. - DOI - PMC - PubMed
- Urkalan K. B.; Sigman M. S. Palladium-Catalyzed Oxidative Intermolecular Difunctionalization of Terminal Alkenes with Organostannanes and Molecular Oxygen. Angew. Chem., Int. Ed. 2009, 48, 3146–3149. 10.1002/anie.200900218. - DOI - PMC - PubMed
- Kalyani D.; Sanford M. S. Oxidatively intercepting Heck intermediates: Pd-catalyzed 1,2-and 1,1-arylhalogenation of alkenes. J. Am. Chem. Soc. 2008, 130, 2150–2151. 10.1021/ja0782798. - DOI - PubMed
-
- García-Domínguez A.; Li Z.; Nevado C. Nickel-Catalyzed Reductive Dicarbofunctionalization of Alkenes. J. Am. Chem. Soc. 2017, 139, 6835–6838. 10.1021/jacs.7b03195. - DOI - PubMed
- Kuang Y. L.; Wang X. F.; Anthony D.; Diao T. N. Ni-catalyzed two-component reductive dicarbofunctionalization of alkenes via radical cyclization. Chem. Commun. 2018, 54, 2558–2561. 10.1039/C8CC00358K. - DOI - PubMed
- Jin Y.; Wang C. Ni-catalysed reductive arylalkylation of unactivated alkenes. Chem. Sci. 2019, 10, 1780–1785. 10.1039/C8SC04279A. - DOI - PMC - PubMed
- Shu W.; Garcia-Dominguez A.; Quiros M. T.; Mondal R.; Cardenas D. J.; Nevado C. Ni-Catalyzed Reductive Dicarbofunctionalization of Nonactivated Alkenes: Scope and Mechanistic Insights. J. Am. Chem. Soc. 2019, 141, 13812–13821. 10.1021/jacs.9b02973. - DOI - PubMed
- Zhao L.; Meng X.; Zou Y. F.; Zhao J. S.; Wang L. L.; Zhang L. L.; Wang C. Directed Nickel-Catalyzed Diastereoselective Reductive Difunctionalization of Alkenyl Amines. Org. Lett. 2021, 23, 8516–8521. 10.1021/acs.orglett.1c03210. - DOI - PubMed
- Terao J.; Saito K.; Nii S.; Kambe N.; Sonoda N. Regioselective double alkylation of styrenes with alkyl halides using a titanocene catalyst. J. Am. Chem. Soc. 1998, 120, 11822–11823. 10.1021/ja982732l. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

