Oxidants and Antioxidants in the Redox Biochemistry of Human Red Blood Cells
- PMID: 36643550
- PMCID: PMC9835686
- DOI: 10.1021/acsomega.2c06768
Oxidants and Antioxidants in the Redox Biochemistry of Human Red Blood Cells
Abstract
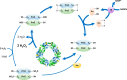
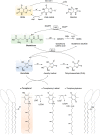
Red blood cells (RBCs) are exposed to both external and internal sources of oxidants that challenge their integrity and compromise their physiological function and supply of oxygen to tissues. Autoxidation of oxyhemoglobin is the main source of endogenous RBC oxidant production, yielding superoxide radical and then hydrogen peroxide. In addition, potent oxidants from other blood cells and the surrounding endothelium can reach the RBCs. Abundant and efficient enzymatic systems and low molecular weight antioxidants prevent most of the damage to the RBCs and also position the RBCs as a sink of vascular oxidants that allow the body to maintain a healthy circulatory system. Among the antioxidant enzymes, the thiol-dependent peroxidase peroxiredoxin 2, highly abundant in RBCs, is essential to keep the redox balance. A great part of the RBC antioxidant activity is supported by an active glucose metabolism that provides reducing power in the form of NADPH via the pentose phosphate pathway. There are several RBC defects and situations that generate oxidative stress conditions where the defense mechanisms are overwhelmed, and these include glucose-6-phosphate dehydrogenase deficiencies (favism), hemoglobinopathies like sickle cell disease and thalassemia, as well as packed RBCs for transfusion that suffer from storage lesions. These oxidative stress-associated pathologies of the RBCs underline the relevance of redox balance in these anucleated cells that lack a mechanism of DNA-inducible antioxidant response and rely on a complex and robust network of antioxidant systems.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Yawata Y.The Red Blood Cell as a Model. In Cell Membrane; Wiley, 2006.
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

