Collective Variables for Crystallization Simulations-from Early Developments to Recent Advances
- PMID: 36643553
- PMCID: PMC9835087
- DOI: 10.1021/acsomega.2c06310
Collective Variables for Crystallization Simulations-from Early Developments to Recent Advances
Abstract
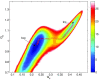
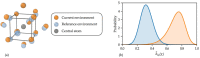
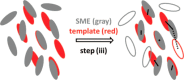
Crystallization is an important physicochemical process which has relevance in material science, biology, and the environment. Decades of experimental and theoretical efforts have been made to understand this fundamental symmetry-breaking transition. While experiments provide equilibrium structures and shapes of crystals, they are limited to unraveling how molecules aggregate to form crystal nuclei that subsequently transform into bulk crystals. Computer simulations, mainly molecular dynamics (MD), can provide such microscopic details during the early stage of a crystallization event. Crystallization is a rare event that takes place in time scales much longer than a typical equilibrium MD simulation can sample. This inadequate sampling of the MD method can be easily circumvented by the use of enhanced sampling (ES) simulations. In most of the ES methods, the fluctuations of a system's slow degrees of freedom, called collective variables (CVs), are enhanced by applying a bias potential. This transforms the system from one state to the other within a short time scale. The most crucial part of such CV-based ES methods is to find suitable CVs, which often needs intuition and several trial-and-error optimization steps. Over the years, a plethora of CVs has been developed and applied in the study of crystallization. In this review, we provide a brief overview of CVs that have been developed and used in ES simulations to study crystallization from melt or solution. These CVs can be categorized mainly into four types: (i) spherical particle-based, (ii) molecular template-based, (iii) physical property-based, and (iv) CVs obtained from dimensionality reduction techniques. We present the context-based evolution of CVs, discuss the current challenges, and propose future directions to further develop effective CVs for the study of crystallization of complex systems.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





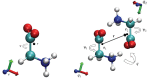









Similar articles
-
Operando Modeling of Zeolite-Catalyzed Reactions Using First-Principles Molecular Dynamics Simulations.ACS Catal. 2023 Aug 15;13(17):11455-11493. doi: 10.1021/acscatal.3c01945. eCollection 2023 Sep 1. ACS Catal. 2023. PMID: 37671178 Free PMC article. Review.
-
OneOPES, a Combined Enhanced Sampling Method to Rule Them All.J Chem Theory Comput. 2023 Sep 12;19(17):5731-5742. doi: 10.1021/acs.jctc.3c00254. Epub 2023 Aug 21. J Chem Theory Comput. 2023. PMID: 37603295 Free PMC article.
-
Reweighted Manifold Learning of Collective Variables from Enhanced Sampling Simulations.J Chem Theory Comput. 2022 Dec 13;18(12):7179-7192. doi: 10.1021/acs.jctc.2c00873. Epub 2022 Nov 11. J Chem Theory Comput. 2022. PMID: 36367826 Free PMC article.
-
Collective Variable-Based Enhanced Sampling: From Human Learning to Machine Learning.J Phys Chem Lett. 2024 Feb 15;15(6):1774-1783. doi: 10.1021/acs.jpclett.3c03542. Epub 2024 Feb 8. J Phys Chem Lett. 2024. PMID: 38329095 Review.
-
Anisotropic Collective Variables with Machine Learning Potential for Ab Initio Crystallization of Complex Ceramics.ACS Nano. 2023 Jul 25;17(14):14099-14113. doi: 10.1021/acsnano.3c04602. Epub 2023 Jul 17. ACS Nano. 2023. PMID: 37458408
Cited by
-
Machine Learning Classification of Local Environments in Molecular Crystals.J Chem Theory Comput. 2024 Jul 23;20(14):6197-6206. doi: 10.1021/acs.jctc.4c00418. Epub 2024 Jul 3. J Chem Theory Comput. 2024. PMID: 38959410 Free PMC article.
-
Spectral Map: Embedding Slow Kinetics in Collective Variables.J Phys Chem Lett. 2023 Jun 8;14(22):5216-5220. doi: 10.1021/acs.jpclett.3c01101. Epub 2023 Jun 1. J Phys Chem Lett. 2023. PMID: 37260045 Free PMC article.
-
Selecting High-Dimensional Representations of Physical Systems by Reweighted Diffusion Maps.J Phys Chem Lett. 2023 Mar 23;14(11):2778-2783. doi: 10.1021/acs.jpclett.3c00265. Epub 2023 Mar 10. J Phys Chem Lett. 2023. PMID: 36897996 Free PMC article.
-
Exploring Carbamazepine Polymorph Crystal Growth in Water by Enhanced Sampling Simulations.ACS Omega. 2024 Aug 16;9(34):36718-36731. doi: 10.1021/acsomega.4c05458. eCollection 2024 Aug 27. ACS Omega. 2024. PMID: 39220538 Free PMC article.
-
Theoretical Investigation into Polymorphic Transformation between β-HMX and δ-HMX by Finite Temperature String.Molecules. 2024 Oct 11;29(20):4819. doi: 10.3390/molecules29204819. Molecules. 2024. PMID: 39459188 Free PMC article.
References
-
- Torrie G. M.; Valleau J. P. Nonphysical sampling distributions in Monte Carlo free-energy estimation: Umbrella sampling. J. Comput. Phys. 1997, 23, 187–199. 10.1016/0021-9991(77)90121-8. - DOI
-
- Nakajima N.; Nakamura H.; Kidera A. Multicanonical ensemble generated by molecular dynamics simulation for enhanced conformational sampling of peptides. J. Phys. Chem. B 1997, 101, 817–824. 10.1021/jp962142e. - DOI
-
- Sugita Y.; Okamoto Y. Replica-exchange molecular dynamics method for protein folding. Chemical physics letters 1999, 314, 141–151. 10.1016/S0009-2614(99)01123-9. - DOI
-
- Sorensen M. R.; Voter A. F. Temperature-accelerated dynamics for simulation of infrequent events. J. Chem. Phys. 2000, 112, 9599–9606. 10.1063/1.481576. - DOI
Publication types
LinkOut - more resources
Full Text Sources

