Adolescent alcohol exposure reduces dopamine 1 receptor modulation of prelimbic neurons projecting to the nucleus accumbens and basolateral amygdala
- PMID: 36643604
- PMCID: PMC9836047
- DOI: 10.1016/j.addicn.2022.100044
Adolescent alcohol exposure reduces dopamine 1 receptor modulation of prelimbic neurons projecting to the nucleus accumbens and basolateral amygdala
Abstract
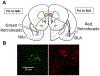
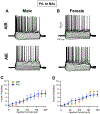
Binge drinking during adolescence is highly prevalent despite increasing evidence of its long-term impact on behaviors associated with modulation of behavioral flexibility by the medial prefrontal cortex (mPFC). In the present study, male and female rats underwent adolescent intermittent ethanol (AIE) exposure by vapor inhalation. After aging to adulthood, retrograde bead labelling and viral tagging were used to identify populations of neurons in the prelimbic region (PrL) of the mPFC that project to specific subcortical targets. Electrophysiological recording from bead-labelled neurons in PrL slices revealed that AIE did not alter the intrinsic excitability of PrL neurons that projected to either the NAc or the BLA. Similarly, recordings of spontaneous inhibitory and excitatory post-synaptic currents revealed no AIE-induced changes in synaptic drive onto either population of projection neurons. In contrast, AIE exposure was associated with a loss of dopamine receptor 1 (D1), but no change in dopamine receptor 2 (D2), modulation of evoked firing of both populations of projection neurons. Lastly, confocal imaging of proximal and apical dendritic tufts of viral-labelled PrL neurons that projected to the nucleus accumbens (NAc) revealed AIE did not alter the density of dendritic spines. Together, these observations provide evidence that AIE exposure results in disruption of D1 receptor modulation of PrL inputs to at least two major subcortical target regions that have been implicated in AIE-induced long-term changes in behavioral control.
Keywords: Adolescence; Alcohol; Development; Dopamine 1 receptor; Prelimbic cortex; Pyramidal neuron.
Conflict of interest statement
Declaration of Competing Interest The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






Similar articles
-
Binge-Like Alcohol Exposure During Adolescence Disrupts Dopaminergic Neurotransmission in the Adult Prelimbic Cortex.Neuropsychopharmacology. 2017 Apr;42(5):1024-1036. doi: 10.1038/npp.2016.190. Epub 2016 Sep 13. Neuropsychopharmacology. 2017. PMID: 27620551 Free PMC article.
-
Adolescent alcohol exposure promotes mechanical allodynia and alters synaptic function at inputs from the basolateral amygdala to the prelimbic cortex.bioRxiv [Preprint]. 2025 Jan 21:2024.06.17.599360. doi: 10.1101/2024.06.17.599360. bioRxiv. 2025. Update in: Elife. 2025 May 08;13:RP101667. doi: 10.7554/eLife.101667. PMID: 38948749 Free PMC article. Updated. Preprint.
-
Excitatory Projections from the Prefrontal Cortex to Nucleus Accumbens Core D1-MSNs and κ Opioid Receptor Modulate Itch-Related Scratching Behaviors.J Neurosci. 2023 Feb 22;43(8):1334-1347. doi: 10.1523/JNEUROSCI.1359-22.2023. Epub 2023 Jan 18. J Neurosci. 2023. PMID: 36653189 Free PMC article.
-
Mechanisms of Persistent Neurobiological Changes Following Adolescent Alcohol Exposure: NADIA Consortium Findings.Alcohol Clin Exp Res. 2019 Sep;43(9):1806-1822. doi: 10.1111/acer.14154. Epub 2019 Aug 14. Alcohol Clin Exp Res. 2019. PMID: 31335972 Free PMC article. Review.
-
Developing a neuronal model for the pathophysiology of schizophrenia based on the nature of electrophysiological actions of dopamine in the prefrontal cortex.Neuropsychopharmacology. 1999 Aug;21(2):161-94. doi: 10.1016/S0893-133X(98)00112-2. Neuropsychopharmacology. 1999. PMID: 10432466 Review.
Cited by
-
Voluntary alcohol consumption during distinct phases of adolescence differentially alters adult fear acquisition, extinction and renewal in male and female rats.Stress. 2023 Nov;26(1):2278315. doi: 10.1080/10253890.2023.2278315. Epub 2023 Nov 5. Stress. 2023. PMID: 37916300 Free PMC article.
-
Adolescent alcohol exposure promotes mechanical allodynia and alters synaptic function at inputs from the basolateral amygdala to the prelimbic cortex.Elife. 2025 May 8;13:RP101667. doi: 10.7554/eLife.101667. Elife. 2025. PMID: 40338067 Free PMC article.
-
Alcohol consumption modulates prelimbic cortex response to cocaine following sequential cocaine and alcohol polysubstance use in the rat.Front Pharmacol. 2023 Mar 16;14:1132689. doi: 10.3389/fphar.2023.1132689. eCollection 2023. Front Pharmacol. 2023. PMID: 37007027 Free PMC article.
-
Adolescent Alcohol Exposure Dysregulates Developing Cortical GABA Circuits.Adv Exp Med Biol. 2025;1473:159-177. doi: 10.1007/978-3-031-81908-7_8. Adv Exp Med Biol. 2025. PMID: 40128479 Review.
-
Adolescent alcohol exposure disrupts episodic-like memory by impairing dopamine synapses in the mouse prelimbic cortex.Neuropharmacology. 2025 Mar 1;265:110255. doi: 10.1016/j.neuropharm.2024.110255. Epub 2024 Dec 4. Neuropharmacology. 2025. PMID: 39643240
References
-
- SAMHSA, Report to Congress on the prevention and reduction of underage drinking, Substance Abuse and Mental Health Services Administration (SAMHSA), US Department of Health and Human Services (HHS), 2018. https://www.stopalcoholabuse.gov/media/ReportToCongress/2018/report_main....
-
- Guttmannova K, Bailey JA, Hill KG, Lee JO, Hawkins JD, Woods ML, Catalano RF, Sensitive periods for adolescent alcohol use initiation: predicting the lifetime occurrence and chronicity of alcohol problems in adulthood, J. Stud. Alcohol. Drugs 72 (2) (2011) 221–231, doi:10.15288/jsad.2011.72.221. - DOI - PMC - PubMed
-
- Mota N, Parada M, Crego A, Doallo S, Caamano-Isorna F, Rodriguez Holguin S, Cadaveira F, Corral M, Binge drinking trajectory and neuropsychological functioning among university students: a longitudinal study, Drug Alcohol. Depend. 133 (1) (2013) 108–114, doi:10.1016/j.drugalcdep.2013.05.024. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
