Differences in histomorphology and expression of key lipid regulated genes of four adipose tissues from Tibetan pigs
- PMID: 36643642
- PMCID: PMC9835692
- DOI: 10.7717/peerj.14556
Differences in histomorphology and expression of key lipid regulated genes of four adipose tissues from Tibetan pigs
Abstract
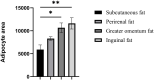
Tibetan pigs, an indigenous pig breed in China, have high overall fat deposition and flavorful and tasty meat. They are thus good models for studying adipogenesis. Few studies have been conducted focusing on expression of lipid regulated genes in different adipose tissues of Tibetan pigs. Therefore, we compared the difference of histomorphology and expression level of lipid regulated genes through qPCR and western blot in subcutaneous fat, perirenal fat, omental adipose tissue, and inguinal fat of Tibetan pigs. Our results showed that the area of subcutaneous adipocytes in Tibetan pigs was smaller, while the other three adipose tissues (perirenal fat, greater omentum fat, inguinal fat) had cell areas of similar size. The gene expression of FABP4, FASN, FABP3, and ME1 in subcutaneous fat was significantly higher than that in perirenal fat. Furthermore, the protein expression of FABP4 was significantly lower in subcutaneous fat than in perirenal fat (p < 0.05), and the expression of FASN was higher in greater omentum fat than in subcutaneous fat (p = 0.084). The difference in adipocyte cell size and expression of lipid-regulated genes in adipose tissues from the various parts of the pig body is likely due to the different cellular lipid metabolic processes. Specially, FABP4 and FASN may be involved in the regulation of fat deposition in different adipose tissues of Tibetan pigs.
Keywords: Adipocyte; C/EBPβ; FABP; Greater omentum fat; Inguinal fat; PPARγ; Perirenal fat; Subcutaneous white adipose tissue; Tibetan pig.
© 2023 Lin et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


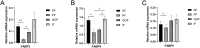


Similar articles
-
Analysis of Transcriptome Differences Between Subcutaneous and Intramuscular Adipose Tissue of Tibetan Pigs.Genes (Basel). 2025 Feb 20;16(3):246. doi: 10.3390/genes16030246. Genes (Basel). 2025. PMID: 40149398 Free PMC article.
-
Single-nucleus RNA sequencing defines adipose tissue subpopulations that contribute to Tibetan pig cold adaptation.BMC Biol. 2025 Apr 24;23(1):107. doi: 10.1186/s12915-025-02211-0. BMC Biol. 2025. PMID: 40275312 Free PMC article.
-
Identification and expression patterns of adipokine genes during adipocyte differentiation in the Tibetan goat (Capra hircus).Gene. 2018 Feb 15;643:17-25. doi: 10.1016/j.gene.2017.11.069. Epub 2017 Nov 29. Gene. 2018. PMID: 29197590
-
Conjugated linoleic acids inhibit lipid deposition in subcutaneous adipose tissue and alter lipid profiles in serum of pigs.J Anim Sci. 2023 Jan 3;101:skad294. doi: 10.1093/jas/skad294. J Anim Sci. 2023. PMID: 37646838 Free PMC article.
-
The origin and purpose of layers of subcutaneous adipose tissue in pigs and man.Horm Mol Biol Clin Investig. 2018 Mar 16;33(1). doi: 10.1515/hmbci-2018-0001. Horm Mol Biol Clin Investig. 2018. PMID: 29547390 Review.
Cited by
-
Identification of potential tissue-specific biomarkers involved in pig fat deposition through integrated bioinformatics analysis and machine learning.Heliyon. 2024 May 15;10(10):e31311. doi: 10.1016/j.heliyon.2024.e31311. eCollection 2024 May 30. Heliyon. 2024. PMID: 38807889 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

