An inexpensive and rapid diagnostic method for detection of SARS-CoV-2 RNA by loop-mediated isothermal amplification (LAMP)
- PMID: 36643803
- PMCID: PMC9831977
- DOI: 10.1016/j.mex.2023.102011
An inexpensive and rapid diagnostic method for detection of SARS-CoV-2 RNA by loop-mediated isothermal amplification (LAMP)
Abstract
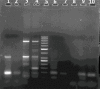
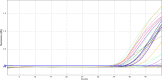
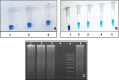
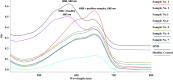
SARS-CoV-2 is a public pandemic health concern globally. Nasopharyngeal and oropharyngeal swab samples are used for Covid-19 viral detection. Sample collection procedure was tedious and uncomfortable and unsuitable for biochemical and CBC analysis in swab samples. Biochemistry and CBC tests are key determinant in management of Covid-19 patients. We developed a LAMP test to detect viral RNA in blood samples. LAMP is required four specific primers targeting the internal transcribed S-region and loop primers for viral RNA amplification. RNA was extracted from blood samples by TRIzol method. LAMP reaction was performed at 60 °C for 1 hour and amplicons were visualized in HNB dye. No cross-reactivity was seen with HBV, HCV, and HIV infected sample. Out of 40 blood samples, 33 samples were positive for LAMP and Q-PCR analysis, one sample was positive for LAMP and negative for Q-PCR, two samples were negative for LAMP but positive for Q-PCR, and four blood samples were negative for LAMP and Q-PCR. LAMP method has an accuracy of 92.50%, with sensitivity and specificity of 94.28% and 80%, respectively. Thus, LAMP diagnostic test has proved reliable, fast, inexpensive and can be useful for detection where the limited resources available.•LAMP method is a potential tool for detection of SARS-CoV-2.•Blood samples are the key determinant for routine diagnostics as well as molecular diagnostics.•LAMP assay is an appropriate diagnostics method which offers greater simplicity, low cost, sensitivity, and specificity than other methods in molecular diagnostics.
Keywords: Blood; CBC; Colorimetric assay; Covid-19; LAMP; Loop Mediated Isothermal Amplification (LAMP); NP; OP; SARS-CoV-2.
© 2023 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Mautner Lena B.A., Christin-Kirsty Baillie, Heike Herold Marie, Wolfram Volkwein, Patrick Guertler, Ute Eberle, Nikolaus Ackermann, Andreas Sing, Melanie Pavlovic, Ottmar Goerlich, Ulrich Busch, Lars Wassill, Ingrid Huber. Rapid point-of-care detection of SARS-CoV-2 using reverse transcription loop-mediated isothermal amplification (RT-LAMP) Virol. J. 2020;17:14. doi: 10.1186/s12985-020-01435-6. - DOI - PMC - PubMed
-
- Amaral C., Antunes W., Moe E., Duarte A.G., Lima L.M.P., Santos C., Gomes I.L., Afonso G.S., Vieira R., Teles H.S.S., Reis M.S., da Silva M.A.R., Henriques A.M., Fevereiro M., Ventura M.R., Serrano M. A molecular test based on RT-LAMP for rapid, sensitive and inexpensive colorimetric detection of SARS-CoV-2 in clinical samples. Sci. Rep. 2021;11:1–12. doi: 10.1038/s41598-021-95799-6. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

