Psychiatric comorbidities and their treatment predict buprenorphine continuation among postpartum people with opioid use disorder
- PMID: 36644222
- PMCID: PMC9835555
- DOI: 10.1016/j.dadr.2022.100121
Psychiatric comorbidities and their treatment predict buprenorphine continuation among postpartum people with opioid use disorder
Abstract
Background: Opioid use disorder (OUD) is a growing crisis among pregnant and postpartum people. Psychiatric comorbidities are common, yet how they impact OUD treatment outcomes is not well characterized. The aim of this study was to assess the association of psychiatric comorbidities and receipt of psychiatric treatment with buprenorphine continuation through one year postpartum among a sample of people with OUD.
Methods: A subsample was identified from a larger retrospective cohort of patients receiving buprenorphine for OUD at the time of delivery from an academic medical center between 2017 and 2020. Medical record abstractions were conducted during pregnancy through one year postpartum. Independent variables included any psychiatric diagnosis and postpartum receipt of psychiatric treatment (medication or behavioral health). The primary outcome was week of buprenorphine discontinuation. Cox Proportional Hazard models were used.
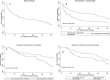
Results: Of 138 patients, 71.8% had a psychiatric condition and 35.5% continued buprenorphine for a full year postpartum. Postpartum buprenorphine continuation was associated with (a) Psychiatric co-morbidity (buprenorphine discontinuation HR 0.49; 95% CI 0.29, 0.82), (b) Receipt of psychiatric medications in weeks 39-52 postpartum (buprenorphine discontinuation HR 0.21; 95% CI 0.06, 0.83), and (c) Receipt of behavioral health therapy in weeks 9-38 postpartum (buprenorphine discontinuation HR 0.40; 95% CI 0.18, 0.90).
Conclusion: Our work suggests a dynamic relationship between OUD treatment outcomes, psychiatric comorbidities and receipt of psychiatric treatments through the highly vulnerable postpartum period. Clinicians and researchers alike should work to advance patient-centered engagement in integrated care models tailored for this unique population.
Keywords: Mental illness; Opioid use disorder; Perinatal; Postpartum.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Methadone and buprenorphine discontinuation among postpartum women with opioid use disorder.Am J Obstet Gynecol. 2021 Oct;225(4):424.e1-424.e12. doi: 10.1016/j.ajog.2021.04.210. Epub 2021 Apr 15. Am J Obstet Gynecol. 2021. PMID: 33845029 Free PMC article.
-
An Integrated Care Model for Pregnant and Postpartum Individuals Receiving Medication for Opioid Use Disorder.J Addict Med. 2023 Mar-Apr 01;17(2):131-139. doi: 10.1097/ADM.0000000000001052. Epub 2022 Aug 17. J Addict Med. 2023. PMID: 35972153 Free PMC article.
-
Concurrent Depression Management in Patients with Opioid Use Disorder Undergoing Buprenorphine Therapy: Association with Buprenorphine Discontinuation.J Dual Diagn. 2025 Apr;21(2):142-151. doi: 10.1080/15504263.2025.2474949. Epub 2025 Apr 1. J Dual Diagn. 2025. PMID: 40168190
-
Prior National Drug Abuse Treatment Clinical Trials Network (CTN) opioid use disorder trials as background and rationale for NIDA CTN-0100 "optimizing retention, duration and discontinuation strategies for opioid use disorder pharmacotherapy (RDD)".Addict Sci Clin Pract. 2021 Mar 6;16(1):15. doi: 10.1186/s13722-021-00223-z. Addict Sci Clin Pract. 2021. PMID: 33676577 Free PMC article. Review.
-
Recommendations for buprenorphine and methadone therapy in opioid use disorder: a European consensus.Expert Opin Pharmacother. 2017 Dec;18(18):1987-1999. doi: 10.1080/14656566.2017.1409722. Epub 2017 Dec 3. Expert Opin Pharmacother. 2017. PMID: 29183228 Review.
References
-
- El-Halabi S., Cooper D.H., Cha D.S., Rosenblat J.D., Gill B., Rodrigues N.B., Lipsitz O., McIntyre R.S., Gill H. The effects of antidepressant medications on antiretroviral treatment adherence in HIV-positive individuals with depression. J. Affect. Disord. 2022;300:219–225. doi: 10.1016/j.jad.2021.12.083. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

