A randomized, double-blind, placebo-controlled phase IIa trial of efruxifermin for patients with compensated NASH cirrhosis
- PMID: 36644237
- PMCID: PMC9832280
- DOI: 10.1016/j.jhepr.2022.100563
A randomized, double-blind, placebo-controlled phase IIa trial of efruxifermin for patients with compensated NASH cirrhosis
Abstract
Background & aims: Efruxifermin has shown clinical efficacy in patients with non-alcoholic steatohepatitis (NASH) and F1-F3 fibrosis. The primary objective of the BALANCED Cohort C was to assess the safety and tolerability of efruxifermin in patients with compensated NASH cirrhosis.
Methods: Patients with NASH and stage 4 fibrosis (n = 30) were randomized 2:1 to receive efruxifermin 50 mg (n = 20) or placebo (n = 10) once-weekly for 16 weeks. The primary endpoint was safety and tolerability of efruxifermin. Secondary and exploratory endpoints included evaluation of non-invasive markers of liver injury and fibrosis, glucose and lipid metabolism, and changes in histology in a subset of patients who consented to end-of-study liver biopsy.
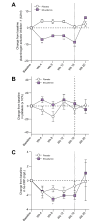
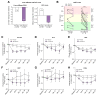
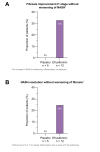
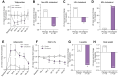
Results: Efruxifermin was safe and well-tolerated; most adverse events (AEs) were grade 1 (n = 7, 23.3%) or grade 2 (n = 19, 63.3%). The most frequent AEs were gastrointestinal, including transient, mild to moderate diarrhea, and/or nausea. Significant improvements were noted in key markers of liver injury (alanine aminotransferase) and glucose and lipid metabolism. Sixteen-week treatment with efruxifermin was associated with significant reductions in non-invasive markers of fibrosis including Pro-C3 (least squares mean change from baseline [LSMCFB] -9 μg/L efruxifermin vs. -3.4 μg/L placebo; p = 0.0130) and ELF score (-0.4 efruxifermin vs. +0.4 placebo; p = 0.0036), with a trend towards reduced liver stiffness (LSMCFB -5.7 kPa efruxifermin vs. -1.1 kPa placebo; n.s.). Of 12 efruxifermin-treated patients with liver biopsy after 16 weeks, 4 (33%) achieved fibrosis improvement of at least one stage without worsening of NASH, while an additional 3 (25%) achieved resolution of NASH, compared to 0 of 5 placebo-treated patients.
Conclusions: Efruxifermin appeared safe and well-tolerated with encouraging improvements in markers of liver injury, fibrosis, and glucose and lipid metabolism following 16 weeks of treatment, warranting confirmation in larger and longer term studies.
Lay summary: Cirrhosis resulting from non-alcoholic steatohepatitis (NASH), the progressive form of non-alcoholic fatty liver disease, represents a major unmet medical need. Currently there are no approved drugs for the treatment of NASH. This proof-of-concept randomized, double-blind clinical trial demonstrated the potential therapeutic benefit of efruxifermin treatment compared to placebo in patients with cirrhosis due to NASH.
Clinical trial number: NCT03976401.
Keywords: ADA(s), anti-drug antibody(ies); AE, adverse event; ALP, alkaline phosphatase; ALT, alanine aminotransferase; ANCOVA, analysis of covariance; AST, aspartate aminotransferase; CFB, change from baseline; CTX-1, C-terminal telopeptide of type 1 collagen; C–P, Child-Pugh; DXA, dual-energy X-ray absorptiometry; ELF, enhanced liver fibrosis; FGF21; FGF21, fibroblast growth factor-21; FGFR, fibroblast growth factor receptor; GGT, gamma-glutamyltransferase; HDL-C, HDL-cholesterol; HOMA-IR, homeostatic model assessment of insulin resistance; HPA, hypothalamic-pituitary-adrenal; HbA1c, hemoglobin A1c; INR, international normalized ratio; IRT, interactive response technology; LDL-C, LDL-cholesterol; LS, least squares; MELD, model for end-stage liver disease; NAFLD, non-alcoholic fatty liver disease; NAS, NAFLD activity score; NASH, non-alcoholic steatohepatitis; NAb, neutralizing antibody; Non-HDL-C, non-HDL-cholesterol; P1NP, procollagen type-I N-terminal propeptide; P3NP, procollagen type III N-terminal propeptide; PAI-1, plasminogen activator inhibitor-1; Pro-C3, N-terminal type III collagen propeptide; TEAE, treatment-emergent adverse event; TIMP-1, tissue inhibitor of metalloproteinase-1; ULN, upper limit of normal; cirrhosis; clinical trial; efruxifermin; histopathology; hs-CRP, high-sensitivity C-reactive protein; liver disease; non-alcoholic steatohepatitis/NASH; nonalcoholic fatty liver disease/NAFLD.
© 2022 The Author(s).
Conflict of interest statement
Stephen A. Harrison holds a leadership or fiduciary role at, advises, consults for, receives grants or contracts from Northsea Therapeutics. He advises, consults for, receives grants or contracts, support for attending meetings and/or travel from Madrigal Pharmaceuticals, Inc. He advises, consults for, and receives grants or contracts from Akero Therapeutics, Inc. He advises, consults for, and receives grants or contracts from Axcella Health, Inc., Cymabay Therapeutics, Inc., Galectin Therapeutics, Inc., Hepion Pharmaceuticals, Inc., Hightide Therapeutics, Inc., Intercept Pharmaceuticals, Inc., Metacrine Inc., NGM Biopharmaceuticals Inc., Genfit Corp, Novo Nordisk, Poxel, Sagimet Biosciences. He advises and receives grants or contracts from Gilead Sciences, Inc., Galmed Research & Dev. LTD., and Novartis Pharmaceuticals Corp. He consults for and receives grants or contracts from Viking Therapeutics, Inc. and Enyo Pharma S.A. He advises and consults for Altimmune, Echosens North America Inc. Foresite Labs, LLC, HistoIndex PTE LTD, Medpace Inc., Prometic, Pharma SMT LTD, Ridgeline and Sonic Incytes Medical Corp, Terns Inc. He advises for 89bio, Arrowhead, Chronwell, CiVi, Indalo, PathAI, and Theratechnologies. He consults for AgomAB, Alentis Therapeutics AG, Alimentiv, Inc, Boston Pharmaceuticals, B Riley FBR Inc. BVF Partners LP, Cohbar, Inc. Canfite, Corcept Therapeutics, Inc, Fibronostics, Fortress Biotech, Inc GNS, Inipharm, Ionis, Kowa Research Institute, Microba, Nutrasource, Perspectum Diagnostics, and Piper Sandler. He receives grants or contracts from Cirius Therapeutics, Inc., and CiVi Biopharma Inc. He holds stock or stock options at Akero Therapeutics, Inc., Chronwell Inc., Cirius Therapeutics, Inc, Galectin Therapeutics, Inc., Genfit Corp, Hepion Pharmaceuticals Inc., HistoIndex PTE LTD, Metacrine Inc., NGM Biopharmaceuticals., Northsea Therapeutics B.V, and Sonic Incytes Medical Corp. Peter J. Ruane: none. Bradley Freilich: none. Guy Neff has received payment or honoraria from Intercept Pharmaceuticals. Rashmee Patil has received grants to institution from 89 Bio, AltImmune, Boehringer Ingelheim, Bristol Myers Squibb, Corcept Therapeutics, Fibronostics, Galectin Therapeutics, Genentech, Gilead, Helio Health, Hepagene, Madrigal Pharmaceuticals, NGMBio, NorthSea Therapeutics, Poxel, Sagimet Biosciences, and Viking Therapeutics. He consults for Intercept Pharmaceuticals. Cynthia Behling has received payment via Pacific Rim Pathology for liver biopsy scoring. She has received grants as a co-investigator under N100 K U01 DK61734. She has received honoraria for lectures from Pfizer and Alimentev. She is a co-chair on NASH CRN Pathology Committee. Chen Hu: none. Reshma Shringarpure, Brittany de Temple, Erica Fong, Erik J. Tillman, Timothy Rolph, Andrew Cheng, and Kitty Yale are employees of Akero Therapeutics and own stock and/or stock options of Akero Therapeutics. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures

References
-
- Matteoni C.A., Younossi Z.M., Gramlich T., Boparai N., Liu Y.C., McCullough A.J. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. - PubMed
-
- Chalasani N., Younossi Z., Lavine J.E., Charlton M., Cusi K., Rinella M., et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver diseases. Hepatology. 2018;67:328–357. - PubMed
-
- Younossi Z.M., Blissett D., Blissett R., Henry L., Stepanova M., Younossi Y., et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology. 2016;64:1577–1586. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

