A Novel Device for Tricuspid Regurgitation Reduction Featuring 3-Dimensional Leaflet and Atraumatic Anchor: Pivot-TR System
- PMID: 36644275
- PMCID: PMC9831928
- DOI: 10.1016/j.jacbts.2022.06.017
A Novel Device for Tricuspid Regurgitation Reduction Featuring 3-Dimensional Leaflet and Atraumatic Anchor: Pivot-TR System
Abstract
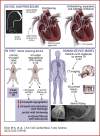
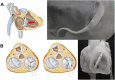
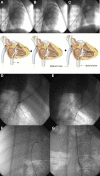
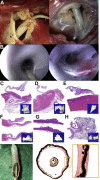
A new device called the Pivot-TR system was designed to treat tricuspid regurgitation with a novel spacer crossing the valve vertically. Its unique atraumatic anchoring system composed of both the elephant long nose and the inferior vena cava spiral anchor, in addition to the relatively easy implantation mechanism, enabled easy retrieval of the system later on. The system showed promising feasibility and safety results in this swine-based animal experiment, which should encourage human translation study.
Keywords: 3D leaflet; AP, anteroposterior; CT, computed tomography; CTI, cavotricuspid isthmus; IVC, inferior vena cava; Pivot-TR system; RA, right atrium; RV, right ventricle; TR, tricuspid regurgitation; TV, tricuspid valve; ePTFE, expanded polytetrafluoroethylene; tricuspid regurgitation; tricuspid valve.
© 2022 The Authors.
Conflict of interest statement
This work was supported by the Korea Medical Device Development Fund grant funded by the South Korean government (Ministry of Science and Information and Communication Technologies, Ministry of Trade, Industry and Energy, Ministry of Health and Welfare, and Ministry of Food and Drug Safety) (project no. RS-2020-KD000104, 1711138183). Tau-PNU Medical provided the Pivot-TR devices. Dr. J.W. Park is a medical consultant for Tau-PNU Medical. Dr. J.H. Kim is an inventor of the Pivot-TR device, is a stockholder of Tau-PNU-Medical; and is clinical director of Tau-PNU Medical. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures


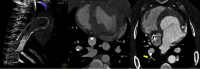
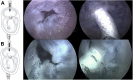
References
-
- Asmarats L., Puri R., Latib A., Navia J.L., Rodés-Cabau J. Transcatheter tricuspid valve interventions: landscape, challenges, and future directions. J Am Coll Cardiol. 2018;71:2935–2956. - PubMed
-
- Enriquez-Sarano M., Messika-Zeitoun D., et al. Tricuspid regurgitation is a public health crisis. Prog Cardiovasc Dis. 2019;62:447–451. - PubMed
-
- Vassileva C.M., Shabosky J., Boley T., Markwell S., Hazelrigg M. Tricuspid valve surgery: the past 10 years from the Nationwide Inpatient Sample (NIS) database. J Thorac Cardiovasc Surg. 2012;143:1043–1049. - PubMed
-
- Kim J.B., Jung S.H., Choo C.H., Chung C.H., Lee D.W. Clinical and echocardiographic outcomes after surgery for severe isolated tricuspid regurgitation. J Thorac Cardiovasc Surg. 2013;146:278–284. - PubMed
LinkOut - more resources
Full Text Sources

