Hyperparathyroidism Is an Independent Risk Factor for Allograft Dysfunction in Pediatric Kidney Transplantation
- PMID: 36644359
- PMCID: PMC9832060
- DOI: 10.1016/j.ekir.2022.10.018
Hyperparathyroidism Is an Independent Risk Factor for Allograft Dysfunction in Pediatric Kidney Transplantation
Abstract
Introduction: Little is known about the consequences of deranged chronic kidney disease-mineral and bone disorder (CKD-MBD) parameters on kidney allograft function in children. We examined a relationship between these parameters over time and allograft outcome.
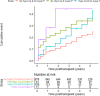
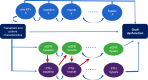
Methods: This registry study from the Cooperative European Paediatric Renal Transplant Initiative (CERTAIN) collected data at baseline, months 1, 3, 6, 9, and 12 after transplant; and every 6 months thereafter up to 5 years. Survival analysis for a composite end point of graft loss or estimated glomerular filtration rate (eGFR) ≤30 ml/min per 1.73 m2 or a ≥50% decline from eGFR at month 1 posttransplant was performed. Associations of parathyroid hormone (PTH), calcium, phosphate, and 25-hydroxyvitamin D (25(OH)D) with allograft outcome were investigated using conventional stratified Cox proportional hazards models and further verified with marginal structural models with time-varying covariates.
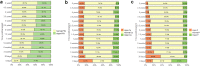
Results: We report on 1210 patients (61% boys) from 16 European countries. The composite end point was reached in 250 grafts (21%), of which 11 (4%) were allograft losses. In the conventional Cox proportional hazards models adjusted for potential confounders, only hyperparathyroidism (hazard ratio [HR], 2.94; 95% confidence interval [CI], 1.82-4.74) and hyperphosphatemia (HR, 1.94; 95% CI, 1.28-2.92) were associated with the composite end point. Marginal structural models showed similar results for hyperparathyroidism (HR, 2.74; 95% CI, 1.71-4.38), whereas hyperphosphatemia was no longer significant (HR, 1.35; 95% CI, 0.87-2.09), suggesting that its association with graft dysfunction can be ascribed to a decline in eGFR.
Conclusion: Hyperparathyroidism is a potential independent risk factor for allograft dysfunction in children.
Keywords: allograft outcome; hyperparathyroidism; kidney transplantation; pediatric; structural marginal models.
© 2022 Published by Elsevier Inc. on behalf of the International Society of Nephrology.
Figures



References
-
- Bonthuis M., Busutti M., van Stralen K.J., et al. Mineral metabolism in European children living with a renal transplant: a European Society for Paediatric Nephrology/European Renal Association-European Dialysis and Transplant Association Registry study. Clin J Am Soc Nephrol. 2015;10:767–775. doi: 10.2215/CJN.06200614. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

