ATR inhibition overcomes platinum tolerance associated with ERCC1- and p53-deficiency by inducing replication catastrophe
- PMID: 36644397
- PMCID: PMC9832712
- DOI: 10.1093/narcan/zcac045
ATR inhibition overcomes platinum tolerance associated with ERCC1- and p53-deficiency by inducing replication catastrophe
Abstract
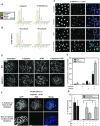
ERCC1/XPF is a heterodimeric DNA endonuclease critical for repair of certain chemotherapeutic agents. We recently identified that ERCC1- and p53-deficient lung cancer cells are tolerant to platinum-based chemotherapy. ATR inhibition synergistically re-stored platinum sensitivity to platinum tolerant ERCC1-deficient cells. Mechanistically we show this effect is reliant upon several functions of ATR including replication fork protection and altered cell cycle checkpoints. Utilizing an inhibitor of replication protein A (RPA), we further demonstrate that replication fork protection and RPA availability are critical for platinum-based drug tolerance. Dual treatment led to increased formation of DNA double strand breaks and was associated with chromosome pulverization. Combination treatment was also associated with increased micronuclei formation which were capable of being bound by the innate immunomodulatory factor, cGAS, suggesting that combination platinum and ATR inhibition may also enhance response to immunotherapy in ERCC1-deficient tumors. In vivo studies demonstrate a significant effect on tumor growth delay with combination therapy compared with single agent treatment. Results of this study have led to the identification of a feasible therapeutic strategy combining ATR inhibition with platinum and potentially immune checkpoint blockade inhibitors to overcome platinum tolerance in ERCC1-deficient, p53-mutant lung cancers.
© The Author(s) 2023. Published by Oxford University Press on behalf of NAR Cancer.
Figures





References
-
- Bepler G., Kusmartseva I., Sharma S., Gautam A., Cantor A., Sharma A., Simon G. RRM1 modulated in vitro and in vivo efficacy of gemcitabine and platinum in non-small-cell lung cancer. J. Clin. Oncol. 2006; 24:4731–4737. - PubMed
-
- Bepler G., Williams C., Schell M.J., Chen W., Zheng Z., Simon G., Gadgeel S., Zhao X., Schreiber F., Brahmer J. et al. Randomized international phase III trial of ERCC1 and RRM1 expression-based chemotherapy versus gemcitabine/carboplatin in advanced non-small-cell lung cancer. J. Clin. Oncol. 2013; 31:2404–2412. - PMC - PubMed
-
- Olaussen K.A., Dunant A., Fouret P., Brambilla E., André F., Haddad V., Taranchon E., Filipits M., Pirker R., Popper H.H. et al. DNA repair by ERCC1 in non–small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N. Engl. J. Med. 2006; 355:983–991. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

