Effects of a Macroporous Resin Extract of Dendrobium officinale Leaves in Rats with Hyperuricemia Induced by Anthropomorphic Unhealthy Lifestyle
- PMID: 36644440
- PMCID: PMC9839412
- DOI: 10.1155/2023/9990843
Effects of a Macroporous Resin Extract of Dendrobium officinale Leaves in Rats with Hyperuricemia Induced by Anthropomorphic Unhealthy Lifestyle
Abstract
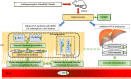
Aim: Hyperuricemia (HUA) has received increased attention in the last few decades due to its global prevalence. Our previous study found that administration of a macroporous resin extract of Dendrobium officinale leaves (DoMRE) to rats with HUA that was induced by exposure to potassium oxazine combined with fructose and a high-purine diet led to a significant reduction in serum uric acid (SUA) levels. The aim of this study was to explore the effects of DoMRE on hyperuricemia induced by anthropomorphic unhealthy lifestyle and to elucidate its possible mechanisms of action.
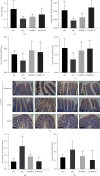
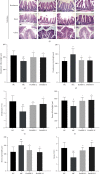
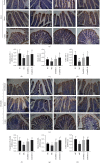
Methods: Dosages (5.0 and 10.0 g/kg/day) of DoMRE were administered to rats daily after induction of HUA by anthropomorphic unhealthy lifestyle for 12 weeks. The levels of UA in the serum, urine, and feces; the levels of creatinine (Cr) in the serum and urine; and the levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) in serum were all measured using an automatic biochemical analyzer. The activities of xanthine oxidase (XOD) and adenosine deaminase (ADA) in the serum, liver, and intestine tissue supernatant were measured using appropriate kits for each biological target. The expressions levels of UA transporters (ABCG2 and GLUT9), tight junction (TJ) proteins (ZO-1 and occludin), and inflammatory factors (IL-6, IL-8, and TNF-α) in the intestine were assayed by immunohistochemical (IHC) staining. Hematoxylin and eosin (H&E) staining was used to assess histological changes in the renal and intestinal tissues.
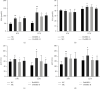
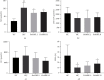
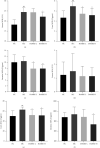
Results: DoMRE treatment significantly reduced SUA levels and concomitantly increased fecal UA (FUA) levels and the fractional excretion of UA (FEUA) in HUA rats. Furthermore, DoMRE significantly reduced both the XOD activity in the serum, liver, and intestine and the ADA activity in the liver and intestine. DoMRE also effectively regulated the expression of GLUT9 and ABCG2 in the intestine, and it significantly upregulated the expression of the intestinal TJ proteins ZO-1 and occludin. Therefore, DoMRE reduced the damage to the intestinal barrier function caused by the increased production of inflammatory factors due to HUA to ensure normal intestinal UA excretion.
Conclusion: DoMRE demonstrated anti-HUA effects in the HUA rat model induced by an anthropomorphic unhealthy lifestyle, and the molecular mechanism appeared to involve the regulation of urate transport-related transporters (ABCG2 and GLUT9) in the intestine, protection of the intestinal barrier function to promote UA excretion, and inhibition of XOD and ADA activity in the liver and intestine to inhibit UA production in the HUA-induced rats.
Copyright © 2023 Lin-Zi Li et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures

References
LinkOut - more resources
Full Text Sources
Research Materials

