Protein phosphatase 2A activators reverse age-related behavioral changes by targeting neural cell senescence
- PMID: 36644807
- PMCID: PMC10014060
- DOI: 10.1111/acel.13780
Protein phosphatase 2A activators reverse age-related behavioral changes by targeting neural cell senescence
Abstract
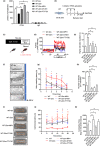
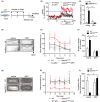
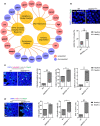
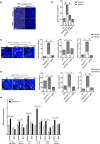
The contribution of cellular senescence to the behavioral changes observed in the elderly remains elusive. Here, we observed that aging is associated with a decline in protein phosphatase 2A (PP2A) activity in the brains of zebrafish and mice. Moreover, drugs activating PP2A reversed age-related behavioral changes. We developed a transgenic zebrafish model to decrease PP2A activity in the brain through knockout of the ppp2r2c gene encoding a regulatory subunit of PP2A. Mutant fish exhibited the behavioral phenotype observed in old animals and premature accumulation of neural cells positive for markers of cellular senescence, including senescence-associated β-galactosidase, elevated levels cdkn2a/b, cdkn1a, senescence-associated secretory phenotype gene expression, and an increased level of DNA damage signaling. The behavioral and cell senescence phenotypes were reversed in mutant fish through treatment with the senolytic ABT263 or diverse PP2A activators as well as through cdkn1a or tp53 gene ablation. Senomorphic function of PP2A activators was demonstrated in mouse primary neural cells with downregulated Ppp2r2c. We conclude that PP2A reduction leads to neural cell senescence thereby contributing to age-related behavioral changes and that PP2A activators have senotherapeutic properties against deleterious behavioral effects of brain aging.
Keywords: Age-related cognitive decline; DNA damage response neural senescence; PPP2R2C; protein phosphatase 2A; senolytics; senotherapy.
© 2023 The Authors. Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures


Similar articles
-
Autophagy regulates cellular senescence by mediating the degradation of CDKN1A/p21 and CDKN2A/p16 through SQSTM1/p62-mediated selective autophagy in myxomatous mitral valve degeneration.Autophagy. 2025 Jul;21(7):1433-1455. doi: 10.1080/15548627.2025.2469315. Epub 2025 Mar 4. Autophagy. 2025. PMID: 39988732 Free PMC article.
-
Apoptotic priming in senescence predicts specific senolysis by quantitative analysis of mitochondrial dependencies.Cell Death Differ. 2025 May;32(5):802-817. doi: 10.1038/s41418-024-01431-1. Epub 2025 Jan 6. Cell Death Differ. 2025. PMID: 39762561
-
Hepatocytes as Model for Investigating Natural Senotherapeutic Compounds and Their Effects on Cell Cycle Dynamics and Genome Stability.Int J Mol Sci. 2025 Jul 16;26(14):6794. doi: 10.3390/ijms26146794. Int J Mol Sci. 2025. PMID: 40725041 Free PMC article. Review.
-
IGFBP-rP1 induces p21 expression through a p53-independent pathway, leading to cellular senescence of MCF-7 breast cancer cells.J Cancer Res Clin Oncol. 2012 Jun;138(6):1045-55. doi: 10.1007/s00432-012-1153-y. Epub 2012 Mar 6. J Cancer Res Clin Oncol. 2012. PMID: 22392074 Free PMC article.
-
Emerging Therapeutic Approaches to Target the Dark Side of Senescent Cells: New Hopes to Treat Aging as a Disease and to Delay Age-Related Pathologies.Cells. 2023 Mar 16;12(6):915. doi: 10.3390/cells12060915. Cells. 2023. PMID: 36980256 Free PMC article. Review.
Cited by
-
Revolutionizing Diabetic Foot Ulcer Care: The Senotherapeutic Approach.Aging Dis. 2024 Apr 16;16(2):946-970. doi: 10.14336/AD.2024.0065. Aging Dis. 2024. PMID: 38739931 Free PMC article. Review.
-
Senotherapeutic repurposing of metformin for age-related diseases and their signaling pathways.Mol Biol Rep. 2025 Apr 22;52(1):410. doi: 10.1007/s11033-025-10524-0. Mol Biol Rep. 2025. PMID: 40261556 Review.
-
Mitochondrial stress orchestrates chromatin remodeling and longevity via phosphoregulation of the NuRD component LIN-40.Sci China Life Sci. 2025 Aug 13. doi: 10.1007/s11427-025-2954-3. Online ahead of print. Sci China Life Sci. 2025. PMID: 40813522
-
Proteomic and phosphoproteomic profilings reveal distinct cellular responses during Tilapinevirus tilapiae entry and replication.PeerJ. 2025 Feb 21;13:e18923. doi: 10.7717/peerj.18923. eCollection 2025. PeerJ. 2025. PMID: 39995988 Free PMC article.
-
Challenges in anti-aging medicine-trends in biomarker discovery and therapeutic interventions for a healthy lifespan.J Cell Mol Med. 2023 Sep;27(18):2643-2650. doi: 10.1111/jcmm.17912. Epub 2023 Aug 23. J Cell Mol Med. 2023. PMID: 37610311 Free PMC article. Review.
References
-
- Acosta, J. C. , Banito, A. , Wuestefeld, T. , Georgilis, A. , Janich, P. , Morton, J. P. , Athineos, D. , Kang, T. W. , Lasitschka, F. , Andrulis, M. , Pascual, G. , Morris, K. J. , Khan, S. , Jin, H. , Dharmalingam, G. , Snijders, A. P. , Carroll, T. , Capper, D. , Pritchard, C. , … Gil, J. (2013). A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nature Cell Biology, 15(8), 978–990. 10.1038/ncb2784 - DOI - PMC - PubMed
-
- Baker, D. J. , Childs, B. G. , Durik, M. , Wijers, M. E. , Sieben, C. J. , Zhong, J. , Saltness, R. A. , Jeganathan, K. B. , Verzosa, G. C. , Pezeshki, A. , Khazaie, K. , Miller, J. D. , & van Deursen, J. (2016). Naturally occurring p16Ink4a‐positive cells shorten healthy lifespan. Nature, 530(7589), 184–189. 10.1038/nature16932 - DOI - PMC - PubMed
-
- Belblidia, H. , Leger, M. , Abdelmalek, A. , Quiedeville, A. , Calocer, F. , Boulouard, M. , Jozet‐Alves, C. , Freret, T. , & Schumann‐Bard, P. (2018). Characterizing age‐related decline of recognition memory and brain activation profile in mice. Experimental Gerontology, 106, 222–231. 10.1016/j.exger.2018.03.006 - DOI - PubMed
-
- Chang, J. , Wang, Y. , Shao, L. , Laberge, R. M. , Demaria, M. , Campisi, J. , Janakiraman, K. , Sharpless, N. E. , Ding, S. , Feng, W. , Luo, Y. , Wang, X. , Aykin‐Burns, N. , Krager, K. , Ponnappan, U. , Hauer‐Jensen, M. , Meng, A. , & Zhou, D. (2016). Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nature Medicine, 22(1), 78–83. 10.1038/nm.4010 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

