Xenotransplantation: Current Challenges and Emerging Solutions
- PMID: 36644844
- PMCID: PMC9846288
- DOI: 10.1177/09636897221148771
Xenotransplantation: Current Challenges and Emerging Solutions
Abstract
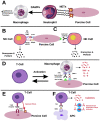
To address the ongoing shortage of organs available for replacement, xenotransplantation of hearts, corneas, skin, and kidneys has been attempted. However, a major obstacle facing xenotransplants is rejection due to a cycle of immune reactions to the graft. Both adaptive and innate immune systems contribute to this cycle, in which natural killer cells, macrophages, and T-cells play a significant role. While advancements in the field of genetic editing can circumvent some of these obstacles, biomarkers to identify and predict xenograft rejection remain to be standardized. Several T-cell markers, such as CD3, CD4, and CD8, are useful in both the diagnosis and prediction of xenograft rejection. Furthermore, an increase in the levels of various circulating DNA markers and microRNAs is also predictive of xenograft rejection. In this review, we summarize recent findings on the advancements in xenotransplantation, with a focus on pig-to-human, the role of immunity in xenograft rejection, and its biomarkers.
Keywords: diagnostic biomarkers; genetic editing; immune rejection; predictive biomarkers; tolerance induction; xenoantigens; xenotransplantation.
Conflict of interest statement
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr. Lerman is an advisor to AstraZeneca, CureSpec, Butterfly Biosciences, Beren Therapeutics, and Ribocure Pharmaceuticals. The authors declare no conflicts of interest.
Figures
References
-
- U.S. Food and Drug Administration. Xenotransplantation. 2021. Accessed June 21, 2022. https://www.fda.gov/vaccines-blood-biologics/xenotransplantation
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

