Endoscopic retrograde cholangiopancreatography and endoscopic ultrasound endoscope reprocessing: Variables impacting contamination risk
- PMID: 36645014
- PMCID: PMC10507511
- DOI: 10.1017/ice.2022.319
Endoscopic retrograde cholangiopancreatography and endoscopic ultrasound endoscope reprocessing: Variables impacting contamination risk
Abstract
Objective: To evaluate variables that affect risk of contamination for endoscopic retrograde cholangiopancreatography and endoscopic ultrasound endoscopes.
Design: Observational, quality improvement study.
Setting: University medical center with a gastrointestinal endoscopy service performing ∼1,000 endoscopic retrograde cholangiopancreatography and ∼1,000 endoscopic ultrasound endoscope procedures annually.
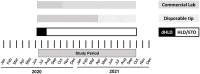
Methods: Duodenoscope and linear echoendoscope sampling (from the elevator mechanism and instrument channel) was performed from June 2020 through September 2021. Operational changes during this period included standard reprocessing with high-level disinfection with ethylene oxide gas sterilization (HLD-ETO) was switched to double high-level disinfection (dHLD) (June 16, 2020-July 15, 2020), and duodenoscopes changed to disposable tip model (March 2021). The frequency of contamination for the co-primary outcomes were characterized by calculated risk ratios.
Results: The overall pathogenic contamination rate was 4.72% (6 of 127). Compared to duodenoscopes, linear echoendoscopes had a contamination risk ratio of 3.64 (95% confidence interval [CI], 0.69-19.1). Reprocessing using HLD-ETO was associated with a contamination risk ratio of 0.29 (95% CI, 0.06-1.54). Linear echoendoscopes undergoing dHLD had the highest risk of contamination (2 of 18, 11.1%), and duodenoscopes undergoing HLD-ETO and the lowest risk of contamination (0 of 53, 0%). Duodenoscopes with a disposable tip had a 0% contamination rate (0 of 27).
Conclusions: We did not detect a significant reduction in endoscope contamination using HLD-ETO versus dHLD reprocessing. Linear echoendoscopes have a risk of contamination similar to that of duodenoscopes. Disposable tips may reduce the risk of duodenoscope contamination.
Conflict of interest statement
All authors report no conflicts of interest relevant to this article.
Figures
References
-
- Preventable tragedies: superbugs and how ineffective monitoring of medical device safety fails patients. US Senate Committee on Health, Education, Labor, and Pensions website. https://www.help.senate.gov/ranking/newsroom/press/preventable-tragedies.... Published January 13, 2016. Accessed January 3, 2023.
-
- Design of Endoscopic Retrograde Cholangiopancreatography (ERCP) duodenoscopes may impede effective cleaning: FDA safety communication. US Food and Drug Administration webiste. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm434871.htm. Published 2015. Accessed March 31, 2015.
-
- Infections associated with reprocessed duodenoscopes. US Food and Drug Administration website. http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/Reprocess.... Published 2016. Accessed January 5, 2017.
-
- FDA recommends healthcare facilities and manufacturers begin transitioning to duodenoscopes with disposable components to reduce risk of patient infection. US Food and Drug Administration webiste. https://www.fda.gov/news-events/press-announcements/fda-recommends-healt.... Published 2019. Accessed January 3, 2023.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical