Induction and Maintenance Treatment With Upadacitinib Improves Health-Related Quality of Life in Patients With Moderately to Severely Active Ulcerative Colitis: Phase 3 Study Results
- PMID: 36645051
- PMCID: PMC10472742
- DOI: 10.1093/ibd/izac260
Induction and Maintenance Treatment With Upadacitinib Improves Health-Related Quality of Life in Patients With Moderately to Severely Active Ulcerative Colitis: Phase 3 Study Results
Abstract
Background: We evaluated the health-related quality of life (HRQoL) benefits of upadacitinib (UPA) induction and maintenance treatment in a phase 3 study of patients with ulcerative colitis (UC) across a broad range of patient-centered outcomes.
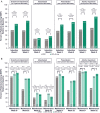
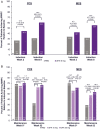
Methods: Patients received UPA 45 mg once daily or placebo as induction treatment for 8 weeks. Patients who achieved clinical response were rerandomized to receive once daily UPA 15 mg, 30 mg, or placebo as maintenance treatment for 52 weeks. The percentages of patients reporting a clinically meaningful within-person change from baseline in the Ulcerative Colitis Symptoms Questionnaire, Inflammatory Bowel Disease Questionnaire, Work Productivity and Impairment Questionnaire, 36-Item Short Form Survey, and European Quality of Life-5 Dimension 5 Levels were evaluated at weeks 2 and 8 of induction and at weeks 0 and 52 of maintenance.
Results: Significant improvements from baseline in all HRQoL measures except the Work Productivity and Impairment Questionnaire-absenteeism were achieved with UPA (P < .001) vs placebo as early as week 2 of induction. These improvements were sustained at week 52 with significantly more patients treated with either 15 mg or 30 mg UPA vs placebo achieving meaningful within-person change in the Ulcerative Colitis Symptoms Questionnaire; Inflammatory Bowel Disease Questionnaire; overall work impairment, presenteeism, and activity impairment; both 36-Item Short Form Survey Physical and Mental Component Summaries; and European Quality of Life-5 Dimension 5 Levels (P < .001).
Conclusions: Induction treatment with UPA 45 mg significantly improved HRQoL measures. A significantly higher percentage of patients who responded to induction treatment with UPA maintained clinically meaningful improvements consistently across a wide range of HRQoL outcomes after 52 weeks of maintenance therapy with UPA (15 mg and 30 mg) compared with placebo. (ClinicalTrials.gov, Numbers: NCT02819635, NCT03653026).
Keywords: clinical trials; quality of life; socioeconomical and psychological endpoints.
Plain language summary
Patients with moderate-to-severe ulcerative colitis who received upadacitinib induction treatment demonstrated clinically meaningful improvements across multiple health-related quality of life assessments, as early as induction week 2, that persisted with maintenance treatment to 52 weeks, compared with placebo.
© 2023 Crohn’s & Colitis Foundation. Published by Oxford University Press on behalf of Crohn’s & Colitis Foundation.
Conflict of interest statement
J.P. has received financial support for research, received lecture fee(s), and/or received consultancy fees from AbbVie, Arena Pharmaceuticals, Athos, Boehringer Ingelheim, Celltrion, Ferring, Galapagos, Genentech, GSK, Janssen, Mirum Pharma, Morphic Therapeutic, Origo, Pandion, Pfizer, Progenity, Revolo Biotherapeutics, Robarts, Roche, Takeda, Theravance, and Wassermann. E.V.L. has received financial support for research and/or consultancy fees from AbbVie, Amgen, Arena, BI, BMS, Calibr, Celgene, Celltrion Healthcare, Fresenius Kabi, Genentech, Gilead, GlaxoSmithKline, Gossamer Bio, Iterative Scopes, Janssen, Lilly, Morphic Therapeutics, Ono Pharma, Pfizer, Protagonist, Receptos, Robarts Clinical Trials, Scipher, Sun, Surrozen, Takeda, Theravance, and UCB. P.D.R.H. has received consultancy fees and/or research funding from AbbVie, the Crohn’s and Colitis Foundation, Eli Lilly, Lycera, the National Institutes of Health, Pfizer, PRIME Medical Education, and Takeda. J.O.L. has served as consultant and on the advisory board for, received speaker fees from, received sponsorship for academic meetings from, and/or received investigator-led research grants from AbbVie, Allergan, Atlantic, BMS, Celgene, Celltrion, Ferring, Galapagos, Gilead, GSK, Janssen, Lilly, MSD, Napp, Norgine, Pfizer, Shire, Takeda, Tillotts, and Vifor Pharma. W.Z., X.Y, D.I., C.P., and Y.S.G. are full-time employees of AbbVie and may hold AbbVie stock and/or stock options. J.T. was a summer intern at AbbVie at the time this study was conducted, is currently an AbbVie contractor and PhD student at University of Washington, and has received financial support from the Agency for Healthcare Research and Quality, National Institutes of Health, ARCS Foundation, and the Washington Research Foundation. S.V. has received grants from AbbVie, J&J, Pfizer, Galapagos, and Takeda; has received consulting and/or speaking fees from AbbVie, AbolerIS Pharma, AgomAb, Alimentiv, Arena Pharmaceuticals, AstraZeneca, Avaxia, BMS, Boehringer Ingelheim, Celgene, CVasThera, Dr. Falk Pharma, Ferring, Galapagos, Genentech-Roche, Gilead, GSK, Hospira, Imidomics, Janssen, J&J, Lilly, Materia Prima, MiroBio, Morphic, MrMHealth, Mundipharma, MSD, Pfizer, Prodigest, Progenity, Prometheus, Robarts Clinical Trials, Second Genome, Shire, Surrozen, Takeda, Theravance, Tillots Pharma AG, and Zealand Pharma.
Figures


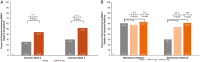
References
-
- Ordás I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ.. Ulcerative colitis. Lancet. 2012;380(9853):1606-1619. - PubMed
-
- Kaplan GG, Ng SC.. Understanding and preventing the global increase of inflammatory bowel disease. Gastroenterology. 2017;152(2):313-321.e2. - PubMed
-
- Armuzzi A, Liguori G.. Quality of life in patients with moderate to severe ulcerative colitis and the impact of treatment: a narrative review. Dig Liver Dis. 2021;53(7):803-808. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

