Inositol 1,4,5-trisphosphate receptor type 2 is associated with the bone-vessel axis in chronic kidney disease-mineral bone disorder
- PMID: 36645057
- PMCID: PMC9848274
- DOI: 10.1080/0886022X.2022.2162419
Inositol 1,4,5-trisphosphate receptor type 2 is associated with the bone-vessel axis in chronic kidney disease-mineral bone disorder
Abstract
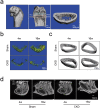
Objective: The pathogenesis of renal osteopathy and cardiovascular disease suggests the disordered bone-vessel axis in chronic kidney disease-mineral bone disorder (CKD-MBD). However, the mechanism of the bone-vessel axis in CKD-MBD remains unclear.Methods: We established a CKD-MBD rat model to observe the pathophysiological phenotype of the bone-vessel axis and performed RNA sequencing of aortas to identify novel targets of the bone-vessel axis in CKD-MBD.Results: The microarchitecture of the femoral trabecular bone deteriorated and alveolar bone loss was aggravated in CKD-MBD rats. The intact parathyroid hormone and alkaline phosphatase levels increased, 1,25-dihydroxyvitamin D3 levels decreased, and intact fibroblast growth factor-23 levels did not increase in CKD-MBD rats at 16 weeks; other bone metabolic parameters in the serum demonstrated dynamic characteristics. With calcium deposition in the abdominal aortas of CKD-MBD rats, RNA sequencing of the aortas revealed a significant decrease in inositol 1,4,5-trisphosphate receptor type 2 (ITPR2) gene levels in CKD-MBD rats. A similar trend was observed in rat aortic smooth muscle cells. As a secretory protein, ITPR2 serum levels decreased at 4 weeks and slightly increased without statistical differences at 16 weeks in CKD-MBD rats. ITPR2 serum levels were significantly increased in patients with vascular calcification, negatively correlated with blood urea nitrogen levels, and positively correlated with serum tartrate-resistant acid phosphatase 5b levels.Conclusion: These findings provide preliminary insights into the role of ITPR2 in the bone-vessel axis in CKD-MBD. Thus, ITPR2 may be a potential target of the bone-vessel axis in CKD-MBD.
Keywords: Bone–vessel axis; chronic kidney disease–mineral bone disorder; clinical relevance; inositol 1,4,5-trisphosphate receptor type 2; vascular calcification.
Conflict of interest statement
An early version of this article was uploaded to a noncommercial preprint server. The following URL is linked to the preprint page:
Figures








References
-
- Zhang L, Wang F, Wang L, et al. . Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet. 2012;379(9818):815–822. - PubMed
-
- Moe S, Drüeke T, Cunningham J, et al. . Definition, evaluation, and classification of renal osteodystrophy: a position statement from kidney disease: improving global outcomes (KDIGO). Kidney Int. 2006;69(11):1945–1953. - PubMed
-
- Cheng X, Nayyar S, Wang M, et al. . Mortality rates among prevalent hemodialysis patients in Beijing: a comparison with USRDS data. Nephrol Dial Transplant. 2013;28(3):724–732. - PubMed
-
- de Jager DJ, Grootendorst DC, Jager KJ, et al. . Cardiovascular and noncardiovascular mortality among patients starting dialysis. JAMA. 2009;302(16):1782–1789. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
