A phase II single-arm trial of memantine for prevention of cognitive decline during chemotherapy in patients with early breast cancer: Feasibility, tolerability, acceptability, and preliminary effects
- PMID: 36645168
- PMCID: PMC10134315
- DOI: 10.1002/cam4.5619
A phase II single-arm trial of memantine for prevention of cognitive decline during chemotherapy in patients with early breast cancer: Feasibility, tolerability, acceptability, and preliminary effects
Abstract
Background: Cognitive difficulties have been described after chemotherapy for breast cancer, but there is no standard of care to improve cognitive outcomes in these patients. This trial examined the feasibility, tolerability, acceptability, and preliminary effects of memantine to prevent cognitive decline during chemotherapy for breast cancer.
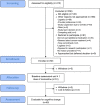
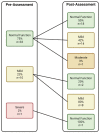
Methods: Patients with stage I-III breast cancer, scheduled for neo/adjuvant chemotherapy, completed a cognitive battery prior to and 4 weeks after completing chemotherapy. Memantine (10 mg BID) was administered concurrent with chemotherapy. Our primary cognitive outcome was visual working memory assessed by the Delayed Matching to Sample test. We used the Brief Medication Questionnaire to assess acceptability.
Results: Of 126 patients approached, 56 (44%) enrolled. Forty-five (80%) received ≥1 dose of memantine and completed pre-post assessments. Seventy-six percent reported taking ≥90% of scheduled doses. Participants were mean age of 56, 77% White, and 57% had stage I disease. Sixty-four percent had stable or improved Delayed Matching to Sample test scores. Stable or improved cognition was observed in 87%-91% across objective cognitive domain composite measures. Sixty-six percent self-reported stable or improved cognitive symptoms. There were seven greater than or equal to grade 3 adverse events; two were possibly related to memantine. Only 5% reported that taking memantine was a disruption to their lives.
Conclusions: Memantine was well-tolerated and consistently taken by a large majority of patients receiving breast cancer chemotherapy. The majority demonstrated stable or improved cognition from pre- to post-assessment. Randomized trials are needed to determine memantine's efficacy to ameliorate cognitive loss.
Trial registration: ClinicalTrials.gov NCT04033419.
Keywords: behavioral science; breast cancer; cancer-related cognitive impairment; clinical trials; cognitive dysfunction; memantine; quality of life.
© 2023 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
Emily Ray receives research funding from OptumHealth and Pfizer. E. Claire Dees consults for Sanofi and receives research funding from Novartis, Genentech/Roche, Pfizer, Merck, H3 Biomedicine, and Meryx Pharmaceuticals. Katherine Reeder‐Hayes receives research funding from Pfizer. Lisa Carey receives research funding from Syndax, Novartis, NanoString Technologies, Abbvie, Seattle Genetics, and Veracyte. Yara Abdou consults for Exact Sciences.
Figures
References
-
- Van Dyk K, Ganz PA. Cancer‐related cognitive impairment in patients with a history of breast cancer. JAMA. 2021;326(17):1736‐1737. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical