Thiophene-Based Ligands for Histological Multiplex Spectral Detection of Distinct Protein Aggregates in Alzheimer's Disease
- PMID: 36645413
- PMCID: PMC10101888
- DOI: 10.1002/chem.202203568
Thiophene-Based Ligands for Histological Multiplex Spectral Detection of Distinct Protein Aggregates in Alzheimer's Disease
Abstract
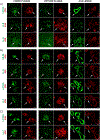
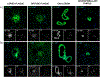
The aggregation and accumulation of proteins in the brain is the defining feature of many devastating neurodegenerative diseases. The development of fluorescent ligands that bind to these accumulations, or deposits, is essential for the characterization of these neuropathological lesions. We report the synthesis of donor-acceptor-donor (D-A-D) thiophene-based ligands with different emission properties. The D-A-D ligands displayed selectivity towards distinct disease-associated protein deposits in histological sections from postmortem brain tissue of individuals affected by Alzheimer's disease (AD). The ability of the ligands to selectively identify AD-associated pathological alterations, such as deposits composed of aggregates of the amyloid-β (Aβ) peptide or tau, was reduced when the chemical composition of the ligands was altered. When combining the D-A-D ligands with conventional thiophene-based ligands, superior spectral separation of distinct protein aggregates in AD tissue sections was obtained. Our findings provide the structural and functional basis for the development of new fluorescent ligands that can distinguish between aggregated proteinaceous species, as well as offer novel strategies for developing multiplex fluorescence detection of protein aggregates in tissue sections.
Keywords: Alzheimer's disease; amyloid-β; fluorescent ligands; protein aggregates; tau.
© 2023 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Figures




References
-
- Ross A, Poirier MA, Nat. Med 2004, 10, S10– S17. - PubMed
-
- Chiti F, Dobson CM, Annu. Rev. Biochem 2006, 75, 333– 366. - PubMed
-
- Benson MD, Buxbaum JN, Eisenberg DS, Merlini G, Saraiva MJM, Sekijima Y, Sipe JD, Westermark P, Amyloid 2020, 27, 217–222. - PubMed
-
- Bennhold H, Muench. Med. Wochenschr 1922, 44,1537 –1538.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

