Steroidal glycoside profile differences among primary roots system and adventitious roots in Solanum dulcamara
- PMID: 36645517
- PMCID: PMC9842586
- DOI: 10.1007/s00425-023-04072-9
Steroidal glycoside profile differences among primary roots system and adventitious roots in Solanum dulcamara
Abstract
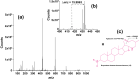
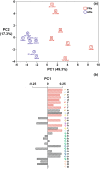
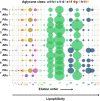
Solanum dulcamara primary and adventitious roots showed qualitative and quantitative differences in their steroidal glycosides profile. This opened new venues to evaluate the bioactivity of these molecules in belowground ecosystems. The Solanum genus is characterized by the presence of steroidal glycosides (SGs) that confer herbivore resistance and serve as drug precursors in the pharmaceutical industry. Solanum dulcamara is a self-compatible, sexually reproducing species that produces seeds after buzz-pollination. In addition, primordia on the stem facilitate clonal propagation via adventitious root (AR) formation. ARs contain aerenchyma being developmentally and morphologically different from primary roots (PRs). Therefore, we hypothesized that ARs and PRs have different SG profiles. Aiming to assess differences in SGs profiles in S. dulcamara roots in relation to their origins and morphologies, we used liquid chromatography coupled to electron spray ionization quadruple time of flight mass spectrometry (LC-ESI-qToF-MS) to profile SGs from PRs and ARs of seven S. dulcamara individuals. Mass fragmentation pattern analysis indicated the presence of 31 SG-type structures, including those with spirostans and furostans moieties. We assigned the 31 structures to 9 classes of steroidal aglycons (SAgls) that differ in hydroxylation and degree of unsaturation. We found that SAgls were conjugated with di-, tri- and tetra saccharides whereby one compound contained a malonylated sugar. Principle component analysis showed that SG profiles of PRs and ARs separated on the first principal component, supporting our hypothesis. Specifically, PRs contain higher number of SGs than ARs with some compounds exclusively present in PRs. Our results reveal a high level of novel chemodiversity in PRs and ARs of Solanum dulcamara. The knowledge gained will deepen our understanding of SGs biosynthesis and their functional role in plant-environment interactions.
Keywords: Chemodiversity; Glycosides; LC–MS; Mass spectrometry; Solanaceae; Steroids.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures

References
-
- Ahmed S, Shevlin P. The possible involvement of excited singlet methylene in the deoxygenation of formaldehyde by atomic carbon. J Am Chem Soc. 1983;105:6488–6490. doi: 10.1021/ja00359a021. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

