Twelve-month end point results from the evaluation of the Zilver Vena venous stent in the treatment of symptomatic iliofemoral venous outflow obstruction (VIVO clinical study)
- PMID: 36646383
- PMCID: PMC12245430
- DOI: 10.1016/j.jvsv.2022.12.066
Twelve-month end point results from the evaluation of the Zilver Vena venous stent in the treatment of symptomatic iliofemoral venous outflow obstruction (VIVO clinical study)
Erratum in
-
Correction.J Vasc Surg Venous Lymphat Disord. 2023 Nov;11(6):1292. doi: 10.1016/j.jvsv.2023.08.001. J Vasc Surg Venous Lymphat Disord. 2023. PMID: 37863555 No abstract available.
Abstract
Background: In the present study, we evaluated the safety and effectiveness of the Zilver Vena venous stent in the treatment of patients with symptomatic iliofemoral outflow obstruction.
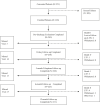
Methods: The VIVO clinical study was a prospective, nonrandomized, multicenter study that enrolled patients with symptomatic obstruction of one iliofemoral venous segment. Included were patients with Clinical, Etiological, Anatomical, Pathophysiological (CEAP) clinical classification of ≥3 or a Venous Clinical Severity Score (VCSS) pain score of ≥2. All patients received a self-expanding venous stent (Zilver Vena venous stent; Cook Ireland Ltd, Limerick, Ireland). The primary safety end point was 30-day freedom from major adverse events. The primary effectiveness end point was the 12-month rate of primary quantitative patency by venography as determined by the core laboratory. The secondary end point was the change in the VCSS from baseline to 1 and 12 months. Additional measures included freedom from clinically driven reintervention; change in the CEAP C classification, Venous Disability Score (VDS), and Chronic Venous Disease Quality of Life Questionnaire (CIVIQ) scores from baseline to 12 months; and stent durability measures.
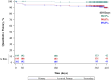
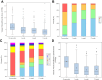
Results: Between December 2013 and October 2016, 243 patients (70% female; mean age, 53 ± 15 years; 67.5% with current or previous deep vein thrombosis) were enrolled at 30 institutions. Iliac vein compression by the iliac artery (n = 191; 78.6%) was the primary indication for stent placement. The mean lesion length was 98.6 ± 69.8 mm. The 30-day freedom from major adverse events rate was 96.7%, greater than the literature-defined performance goal of 87% (95% confidence interval [CI], 93.5%-98.6%; P < .0001). The 12-month primary quantitative patency rate was 89.9%, greater than the literature-defined performance goal of 76% (95% CI, 85.1%-93.4%; P < .0001). The change in the VCSS from baseline was -3.0 (95% CI, -3.5 to -2.6; P < .0001) at 1 month and -4.2 (95% CI, -4.7 to -3.7; P < .0001) at 12 months, demonstrating clinical improvement. Similarly, significantly (P < .0001) fewer symptoms over time (from preprocedure through 12 months) were measured using the clinical measures of VDS, CEAP C classification, and CIVIQ. The 12-month rate of freedom from clinically driven reintervention was 95.8% ± 1.3%. Through 12 months, no stent fractures and one clinical migration (Clinical Events Committee adjudicated the latter as technique-related due to device undersizing at placement) had occurred.
Conclusions: The 12-month results of the VIVO study have demonstrated the safety and effectiveness of the Zilver Vena venous stent for the treatment of symptomatic iliofemoral venous outflow obstruction, including clinical symptom improvement compared with baseline.
Trial registration: ClinicalTrials.gov NCT01970007.
Keywords: Acute deep vein thrombosis; Iliofemoral venous outflow obstruction; Nonthrombotic iliac venous lesions; Post-thrombotic syndrome; Zilver Vena stent.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Figures

Comment in
-
An effective stent for most.J Vasc Surg Venous Lymphat Disord. 2023 May;11(3):542. doi: 10.1016/j.jvsv.2023.01.002. J Vasc Surg Venous Lymphat Disord. 2023. PMID: 37080685 Free PMC article. No abstract available.
References
-
- Neglén P. Stenting is the “method-of-choice” to treat iliofemoral venous outflow obstruction. J Endovasc Ther. 2009;16:492–493. - PubMed
-
- Kölbel T., Lindh M., Åkesson M., Wassèlius J., Gottsäter A., Ivancev K. Chronic iliac vein occlusion: midterm results of endovascular recanalization. J Endovasc Ther. 2009;16:483–491. - PubMed
-
- Razavi M.K., Jaff M.R., Miller L.E. Safety and effectiveness of stent placement for iliofemoral venous outflow obstruction: systematic review and meta-analysis. Circ Cardiovasc Interv. 2015;8:e002772. - PubMed
-
- Razavi M.K., Black S., Gagne P., Chiacchierini R., Nicolini P., Marston W. Pivotal study of endovenous stent placement for symptomatic iliofemoral venous obstruction. Circ Cardiovasc Interv. 2019;12:e008268. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

