Multifunctional Integrated Nanozymes Facilitate Spinal Cord Regeneration by Remodeling the Extrinsic Neural Environment
- PMID: 36646515
- PMCID: PMC9982579
- DOI: 10.1002/advs.202205997
Multifunctional Integrated Nanozymes Facilitate Spinal Cord Regeneration by Remodeling the Extrinsic Neural Environment
Abstract
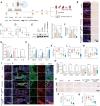
High levels of reactive oxygen species (ROS) and inflammation create a complicated extrinsic neural environment that dominates the initial post-injury period after spinal cord injury (SCI). The compensatory pathways between ROS and inflammation limited the efficacy of modulating the above single treatment regimen after SCI. Here, novel "nanoflower" Mn3 O4 integrated with "pollen" IRF-5 SiRNA was designed as a combination antioxidant and anti-inflammatory treatment after SCI. The "nanoflower" and "pollen" structure was encapsulated with a neutrophil membrane for protective and targeted delivery. Furthermore, valence-engineered nanozyme Mn3 O4 imitated the cascade response of antioxidant enzymes with a higher substrate affinity compared to natural antioxidant enzymes. Nanozymes effectively catalyzed ROS to generate O2 , which is advantageous for reducing oxidative stress and promoting angiogenesis. The screened "pollen" IRF-5 SiRNA could reverse the inflammatory phenotype by reducing interferon regulatory factors-5 (IRF-5) expression (protein level: 73.08% and mRNA level: 63.10%). The decreased expression of pro-inflammatory factors reduced the infiltration of inflammatory cells, resulting in less neural scarring. In SCI rats, multifunctional nanozymes enhanced the proliferation of various neuronal subtypes (motor neurons, interneurons, and sensory neurons) and the recovery of locomotor function, demonstrating that the remodeling of the extrinsic neural environment is a promising strategy to facilitate nerve regeneration.
Keywords: anti-inflammation; antioxidation; catalytic cascade reaction; spinal cord injury.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- a) Ahuja C. S., Wilson J. R., Nori S., Kotter M. R. N., Druschel C., Curt A., Fehlings M. G., Nat. Rev. Dis. Primers 2017, 3, 17018; - PubMed
- b) Warner F. M., Cragg J. J., Jutzeler C. R., Finnerup N. B., Werhagen L., Weidner N., Maier D., Kalke Y. B., Curt A., Kramer J. L. K., J Neurotrauma 2019, 36, 1461; - PubMed
- c) Zipser C. M., Cragg J. J., Guest J. D., Fehlings M. G., Jutzeler C. R., Anderson A. J., Curt A. J. T. L. N., Lancet Neurol. 2022. - PubMed
-
- a) Bains M., Hall E. D., Biochim. Biophys. Acta 2012, 1822, 675; - PMC - PubMed
- b) Fatima G., Sharma V. P., Das S. K., Mahdi A. A., Spinal Cord 2015, 53, 3; - PubMed
- c) Eli I., Lerner D. P., Ghogawala Z., Neurol Clin 2021, 39, 471; - PubMed
- d) Hall E. D., J. E. J. N., NeuroRx 2004, 80, 100; - PMC - PubMed
- e) Springer J. E., Rao R. R., Lim H. R., Cho S. I., Moon G. J., Lee H. Y., Park E. J., Noh J. S., Gwag B. J. J. J. o. n., J Neurotrauma 2010, 27, 139. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
