Intratumoral microbiota: roles in cancer initiation, development and therapeutic efficacy
- PMID: 36646684
- PMCID: PMC9842669
- DOI: 10.1038/s41392-022-01304-4
Intratumoral microbiota: roles in cancer initiation, development and therapeutic efficacy
Abstract
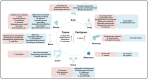
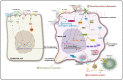
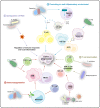
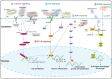
Microorganisms, including bacteria, viruses, fungi, and other eukaryotes, play critical roles in human health. An altered microbiome can be associated with complex diseases. Intratumoral microbial components are found in multiple tumor tissues and are closely correlated with cancer initiation and development and therapy efficacy. The intratumoral microbiota may contribute to promotion of the initiation and progression of cancers by DNA mutations, activating carcinogenic pathways, promoting chronic inflammation, complement system, and initiating metastasis. Moreover, the intratumoral microbiota may not only enhance antitumor immunity via mechanisms including STING signaling activation, T and NK cell activation, TLS production, and intratumoral microbiota-derived antigen presenting, but also decrease antitumor immune responses and promote cancer progression through pathways including upregulation of ROS, promoting an anti-inflammatory environment, T cell inactivation, and immunosuppression. The effect of intratumoral microbiota on antitumor immunity is dependent on microbiota composition, crosstalk between microbiota and the cancer, and status of cancers. The intratumoral microbiota may regulate cancer cell physiology and the immune response by different signaling pathways, including ROS, β-catenin, TLR, ERK, NF-κB, and STING, among others. These viewpoints may help identify the microbiota as diagnosis or prognosis evaluation of cancers, and as new therapeutic strategy and potential therapeutic targets for cancer therapy.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

