CRISPR/Cas9 therapeutics: progress and prospects
- PMID: 36646687
- PMCID: PMC9841506
- DOI: 10.1038/s41392-023-01309-7
CRISPR/Cas9 therapeutics: progress and prospects
Abstract
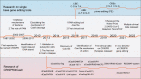
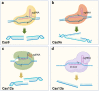
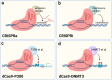
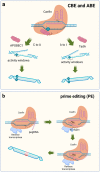
Clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 (Cas9) gene-editing technology is the ideal tool of the future for treating diseases by permanently correcting deleterious base mutations or disrupting disease-causing genes with great precision and efficiency. A variety of efficient Cas9 variants and derivatives have been developed to cope with the complex genomic changes that occur during diseases. However, strategies to effectively deliver the CRISPR system to diseased cells in vivo are currently lacking, and nonviral vectors with target recognition functions may be the focus of future research. Pathological and physiological changes resulting from disease onset are expected to serve as identifying factors for targeted delivery or targets for gene editing. Diseases are both varied and complex, and the choice of appropriate gene-editing methods and delivery vectors for different diseases is important. Meanwhile, there are still many potential challenges identified when targeting delivery of CRISPR/Cas9 technology for disease treatment. This paper reviews the current developments in three aspects, namely, gene-editing type, delivery vector, and disease characteristics. Additionally, this paper summarizes successful examples of clinical trials and finally describes possible problems associated with current CRISPR applications.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

