Hepatic nonvesicular cholesterol transport is critical for systemic lipid homeostasis
- PMID: 36646756
- PMCID: PMC9995220
- DOI: 10.1038/s42255-022-00722-6
Hepatic nonvesicular cholesterol transport is critical for systemic lipid homeostasis
Abstract
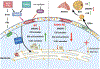
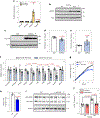
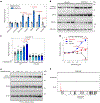
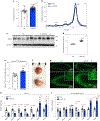
In cell models, changes in the 'accessible' pool of plasma membrane (PM) cholesterol are linked with the regulation of endoplasmic reticulum sterol synthesis and metabolism by the Aster family of nonvesicular transporters; however, the relevance of such nonvesicular transport mechanisms for lipid homeostasis in vivo has not been defined. Here we reveal two physiological contexts that generate accessible PM cholesterol and engage the Aster pathway in the liver: fasting and reverse cholesterol transport. During fasting, adipose-tissue-derived fatty acids activate hepatocyte sphingomyelinase to liberate sequestered PM cholesterol. Aster-dependent cholesterol transport during fasting facilitates cholesteryl ester formation, cholesterol movement into bile and very low-density lipoprotein production. During reverse cholesterol transport, high-density lipoprotein delivers excess cholesterol to the hepatocyte PM through scavenger receptor class B member 1. Loss of hepatic Asters impairs cholesterol movement into feces, raises plasma cholesterol levels and causes cholesterol accumulation in peripheral tissues. These results reveal fundamental mechanisms by which Aster cholesterol flux contributes to hepatic and systemic lipid homeostasis.
© 2023. The Author(s), under exclusive licence to Springer Nature Limited.
Figures










Comment in
-
Asters: rising stars in the cholesterol universe.Nat Metab. 2023 Jan;5(1):16-18. doi: 10.1038/s42255-022-00717-3. Nat Metab. 2023. PMID: 36646755 No abstract available.
-
Aster pathway maintains hepatic cholesterol homeostasis.Nat Rev Endocrinol. 2023 Apr;19(4):188. doi: 10.1038/s41574-023-00812-9. Nat Rev Endocrinol. 2023. PMID: 36792711 No abstract available.
References
-
- Lange Y, Swaisgood MH, Ramos BV & Steck TL Plasma membranes contain half the phospholipid and 90% of the cholesterol and sphingomyelin in cultured human fibroblasts. J. Biol. Chem 264, 3786–3793 (1989). - PubMed
-
- Anderson RA et al. Identification of a form of acyl-CoA: cholesterol acyltransferase specific to liver and intestine in nonhuman primates. J. Biol. Chem 273, 26747–26754 (1998). - PubMed
-
- Pikuleva IA Cholesterol-metabolizing cytochromes P450. Drug Metab. Dispos 34, 513–P520 (2006). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

