Generation of myocyte agonal Ca2+ waves and contraction bands in perfused rat hearts following irreversible membrane permeabilisation
- PMID: 36646772
- PMCID: PMC9842683
- DOI: 10.1038/s41598-023-27807-w
Generation of myocyte agonal Ca2+ waves and contraction bands in perfused rat hearts following irreversible membrane permeabilisation
Abstract
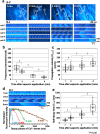
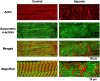
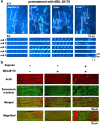
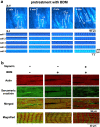
Although irreversible cardiomyocyte injury provokes intracellular Ca2+ ([Ca2+]i) overload, the underlying dynamics of this response and its effects on cellular morphology remain unknown. We therefore visualised rapid-scanning confocal fluo4-[Ca2+]i dynamics and morphology of cardiomyocytes in Langendorff-perfused rat hearts following saponin-membrane permeabilisation. Our data demonstrate that 0.4% saponin-treated myocytes immediately exhibited high-frequency Ca2+ waves (131.3 waves/min/cell) with asynchronous, oscillatory contractions having a mean propagation velocity of 117.8 μm/s. These waves slowly decreased in frequency, developed a prolonged decay phase, and disappeared in 10 min resulting in high-static, fluo4-fluorescence intensity. The myocytes showing these waves displayed contraction bands, i.e., band-like actin-fibre aggregates with disruption of sarcomeric α-actinin. The contraction bands were not attenuated by the abolition of Ca2+ waves under pretreatment with ryanodine plus thapsigargin, but were partially attenuated by the calpain inhibitor MDL28170, while mechanical arrest of the myocytes by 2,3-butanedione monoxime completely attenuated contraction-band formation. The depletion of adenosine 5'-triphosphate by the mitochondrial electron uncoupler carbonyl cyanide 4-trifluoromethoxy phenylhydrazone also attenuated Ca2+ waves and contraction bands. Overall, saponin-induced myocyte [Ca2+]i overload provokes agonal Ca2+ waves and contraction bands. Contraction bands are not the direct consequence of the waves but are caused by cross-bridge interactions of the myocytes under calpain-mediated proteolysis.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

