Molecular residual disease and efficacy of adjuvant chemotherapy in patients with colorectal cancer
- PMID: 36646802
- PMCID: PMC9873552
- DOI: 10.1038/s41591-022-02115-4
Molecular residual disease and efficacy of adjuvant chemotherapy in patients with colorectal cancer
Abstract
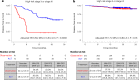
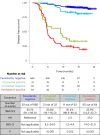
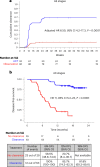
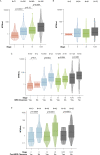
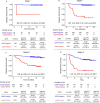
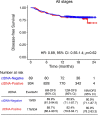
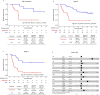
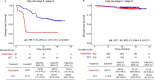
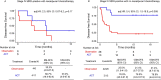
Despite standard-of-care treatment, more than 30% of patients with resectable colorectal cancer (CRC) relapse. Circulating tumor DNA (ctDNA) analysis may enable postsurgical risk stratification and adjuvant chemotherapy (ACT) treatment decision-making. We report results from GALAXY, which is an observational arm of the ongoing CIRCULATE-Japan study (UMIN000039205) that analyzed presurgical and postsurgical ctDNA in patients with stage II-IV resectable CRC (n = 1,039). In this cohort, with a median follow-up of 16.74 months (range 0.49-24.83 months), postsurgical ctDNA positivity (at 4 weeks after surgery) was associated with higher recurrence risk (hazard ratio (HR) 10.0, P < 0.0001) and was the most significant prognostic factor associated with recurrence risk in patients with stage II or III CRC (HR 10.82, P < 0.001). Furthermore, postsurgical ctDNA positivity identified patients with stage II or III CRC who derived benefit from ACT (HR 6.59, P < 0.0001). The results of our study, a large and comprehensive prospective analysis of ctDNA in resectable CRC, support the use of ctDNA testing to identify patients who are at increased risk of recurrence and are likely to benefit from ACT.
© 2023. The Author(s).
Conflict of interest statement
D.K. reports honoraria from Takeda, Chugai, Eli Lilly, MSD, Ono, Taiho, Bristol Myers Squibb, Daiichi-Sankyo, Pfizer, Eisai, Merckbiopharma and Sysmex, and research funding from Ono, MSD, Novartis, Servier, Janssen, IQVIA, Syneoshealth, Cimic and Cimicshiftzero. E.O. reports research funding from Guardant Health, and honoraria from Ono, Takeda, Bayer, Chugai, Taiho, Eli Lilly and Bristol Myers Squibb. Y.N. reports honoraria from Chugai, Merck and Guardant Health AMEA, and research grants from Taiho, Chugai, Guardant Health, Genomedia, Daiichi Sankyo, Seagen and Roche Diagnostics. S.M. reports honoraria from Merck and Roche Diagnostics, and research funding from Roche Diagnostics. H.B. reports research funding from Ono, and honoraria from Ono, Taiho and Eli Lilly. K.Y. reports research funding from Taiho, and honoraria from Takeda, Chugai, Lilly, MSD, Ono, Taiho, Bristol Myers Squibb, Daiichi Sankyo, Merck and Bayer. J.W. reports honoraria for lectures from Johnson & Johnson KK, Medtronic and Eli Lilly, and research funding from Medtronic, AMCO and Terumo outside the submitted work. M.K. reports honoraria from Takeda, Chugai, Lilly, Yakult Honsya and Taiho. K.K. reports honoraria from Takeda, Lilly, Merck and Taiho. H.T. reports honoraria from Chugai, Takeda, Eli Lilly and Merck, and research grants from Taiho, Takeda, Daiichi Sankyo and Roche Diagnostics. I.T. reports honoraria for lectures from Johnson & Johnson KK, Medtronic and Eli Lilly, and research funding from Medtronic and Striker outside the submitted work. T.K. reports honoraria from Chugai, Takeda, Ono, Eli Lilly, Yakult Honsha and Taiho, and research funding from Chugai. T.Y. reports honoraria from Taiho, Chugai, Eli Lilly, Merck, Bayer Yakuhin, Ono and MSD, and research funding from Ono, Sanofi, Daiichi Sankyo, Parexel, Pfizer, Taiho, MSD, Amgen, Genomedia, Sysmex, Chugai and Nippon Boehringer Ingelheim. S.S., V.N.A. and A.A. are full-time employees of Natera with stocks and options to own stock in the company. The remaining authors declare no competing interests.
Figures

Comment in
-
Postoperative circulating tumor DNA could guide CRC adjuvant treatment.Nat Med. 2023 Jan;29(1):39-40. doi: 10.1038/s41591-022-02119-0. Nat Med. 2023. PMID: 36646801 No abstract available.
-
Early MRD predicts disease recurrence and benefit from adjuvant chemotherapy in CRC.Nat Rev Clin Oncol. 2023 Mar;20(3):137. doi: 10.1038/s41571-023-00737-2. Nat Rev Clin Oncol. 2023. PMID: 36717746 No abstract available.
-
Post-surgical ctDNA in patients with CRC.Nat Rev Gastroenterol Hepatol. 2023 Mar;20(3):131. doi: 10.1038/s41575-023-00752-9. Nat Rev Gastroenterol Hepatol. 2023. PMID: 36755081 No abstract available.
References
-
- National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology: Colon Cancer, Vol. 1 (2022).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

