Role of ST6GAL1 and ST6GAL2 in subversion of cellular signaling during enteroaggregative Escherichia coli infection of human intestinal epithelial cell lines
- PMID: 36646912
- PMCID: PMC9843105
- DOI: 10.1007/s00253-022-12321-2
Role of ST6GAL1 and ST6GAL2 in subversion of cellular signaling during enteroaggregative Escherichia coli infection of human intestinal epithelial cell lines
Abstract
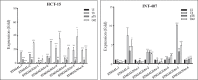
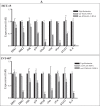
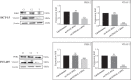
Emerging evidence have suggested that aberrant sialylation on cell-surface carbohydrate architecture may influence host-pathogen interactions. The α2,6-sialyltransferase (ST) enzymes were found to alter the glycosylation pattern of the pathogen-infected host cell-surface proteins, which could facilitate its invasion. In this study, we assessed the role of specific α2,6-ST enzymes in the regulation of enteroaggregative E. coli (EAEC)-induced cell signaling pathways in human intestinal epithelial cells. EAEC-induced expression of α2,6-ST family genes in HCT-15 and INT-407 cell lines was assessed at mRNA level by qRT-PCR. Specific esi-RNA was used to silence the target ST-gene in each of the EAEC-infected cell type. Subsequently, the role of these enzymes in regulation of EAEC-induced cell signaling pathways was unraveled by analyzing the expression of MAPkinases (ERK1/2, p38, JNK) and transcription factors (NFκB, cJun, cFos, STAT) at mRNA and protein levels by qRT-PCR and western immunoblotting, respectively, expression of selected sialoglycoproteins by western immunoblotting along with the secretory IL-8 response using sandwich ELISA. ST6GAL-1 and ST6GAL-2 were efficiently silenced in EAEC-infected HCT-15 and INT-407 cells, respectively. Significant reduction in EAEC-induced activation of MAPKs, transcription factors, sialoglycoproteins, and IL-8 secretion was noted in ST-silenced cells in comparison to the respective control cells. We propose that ST6GAL-1 and ST6GAL-2 are quintessential for EAEC-induced stimulation of MAPK-mediated pathways, resulting in activation of transcription factors, leading to an inflammatory response in the human intestinal epithelial cells. Our study may be helpful to design better therapeutic strategies to control EAEC- infection. KEY POINTS: • EAEC induces α2,6-sialyltransferase (ST) upregulation in intestinal epithelial cells • Target STs (ST6GAL-1 & ST6GAL-2) were efficiently silenced using specific esiRNAs • Expression of MAPKs, transcription factors & IL-8 was reduced in ST silenced cells.
Keywords: Enteroaggregative Escherichia coli; IL-8 secretion; P-3Fax-Neu5Ac; Sialyltransferases; Silencing; qRT-PCR.
© 2023. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
The authors declare no competing interests.
Figures






Similar articles
-
Enteroaggregative Escherichia coli infection induces IL-8 production via activation of mitogen-activated protein kinases and the transcription factors NF-kappaB and AP-1 in INT-407 cells.Mol Cell Biochem. 2010 Apr;337(1-2):17-24. doi: 10.1007/s11010-009-0282-3. Epub 2009 Oct 24. Mol Cell Biochem. 2010. PMID: 19898747
-
Enteroaggregative Escherichia coli induced activation of epidermal growth factor receptor contributes to IL-8 secretion by cultured human intestinal epithelial cells.Microbes Infect. 2023 Sep-Oct;25(7):105166. doi: 10.1016/j.micinf.2023.105166. Epub 2023 Jun 7. Microbes Infect. 2023. PMID: 37290638
-
Enteroaggregative Escherichia coli flagellin-induced interleukin-8 secretion requires Toll-like receptor 5-dependent p38 MAP kinase activation.Immunology. 2004 Aug;112(4):651-60. doi: 10.1111/j.1365-2567.2004.01923.x. Immunology. 2004. PMID: 15270737 Free PMC article.
-
Enteroaggregative Escherichia coli an emergent pathogen with different virulence properties.Rev Latinoam Microbiol. 2005 Jul-Dec;47(3-4):140-59. Rev Latinoam Microbiol. 2005. PMID: 17061538 Review.
-
Autotransporters and virulence of enteroaggregative E. coli.Gut Microbes. 2011 Jan-Feb;2(1):13-24. doi: 10.4161/gmic.2.1.14933. Gut Microbes. 2011. PMID: 21637014 Review.
Cited by
-
Comprehensive analysis of sialylation-related genes and construct the prognostic model in sepsis.Sci Rep. 2024 Aug 5;14(1):18110. doi: 10.1038/s41598-024-69185-x. Sci Rep. 2024. PMID: 39103477 Free PMC article.
-
Dynamic Interactions between Diarrhoeagenic Enteroaggregative Escherichia coli and Presumptive Probiotic Bacteria: Implications for Gastrointestinal Health.Microorganisms. 2023 Dec 8;11(12):2942. doi: 10.3390/microorganisms11122942. Microorganisms. 2023. PMID: 38138086 Free PMC article.
References
-
- Caballero I, Ringot-Destrez B, Si-Tahar M, Barbry P, Guillon A, Lantier I, Berri M, Chevaleyre C, Fleurot I, Barc C, Ramphal R, Pons N, Paquet A, Lebrigand K, Baron C, Bähr A, Klymiuk N, Léonard R, Robbe-Masselot C. Evidence of early increased sialylation of airway mucins and defective mucociliary clearance in CFTR-deficient piglets. J Cyst Fibros. 2021;20(1):173–182. doi: 10.1016/j.jcf.2020.09.009. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

