Clinico-pathological and epigenetic heterogeneity of diffuse gliomas with FGFR3::TACC3 fusion
- PMID: 36647073
- PMCID: PMC9843943
- DOI: 10.1186/s40478-023-01506-z
Clinico-pathological and epigenetic heterogeneity of diffuse gliomas with FGFR3::TACC3 fusion
Abstract
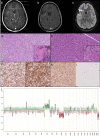
Background: Gliomas with FGFR3::TACC3 fusion mainly occur in adults, display pathological features of glioblastomas (GB) and are usually classified as glioblastoma, IDH-wildtype. However, cases demonstrating pathological features of low-grade glioma (LGG) lead to difficulties in classification and clinical management. We report a series of 8 GB and 14 LGG with FGFR3:TACC3 fusion in order to better characterize them.
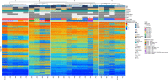
Methods: Centralized pathological examination, search for TERT promoter mutation and DNA-methylation profiling were performed in all cases. Search for prognostic factors was done by the Kaplan-Meir method.
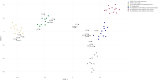
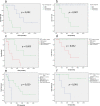
Results: TERT promoter mutation was recorded in all GB and 6/14 LGG. Among the 7 cases with a methylation score > 0.9 in the classifier (v12.5), 2 were classified as glioblastoma, 4 as ganglioglioma (GG) and 1 as dysembryoplastic neuroepithelial tumor (DNET). t-SNE analysis showed that the 22 cases clustered into three groups: one included 12 cases close to glioblastoma, IDH-wildtype methylation class (MC), 5 cases each clustered with GG or DNET MC but none with PLNTY MC. Unsupervised clustering analysis revealed four groups, two of them being clearly distinct: 5 cases shared age (< 40), pathological features of LGG, lack of TERT promoter mutation, FGFR3(Exon 17)::TACC3(Exon 10) fusion type and LGG MC. In contrast, 4 cases shared age (> 40), pathological features of glioblastoma, and were TERT-mutated. Relevant factors associated with a better prognosis were age < 40 and lack of TERT promoter mutation.
Conclusion: Among gliomas with FGFR3::TACC3 fusion, age, TERT promoter mutation, pathological features, DNA-methylation profiling and fusion subtype are of interest to determine patients' risk.
Keywords: 2021 WHO classification of CNS tumours; DNA-methylation profiling; FGFR3:TACC3 fusion; Glioblastoma; Pediatric low grade glioma.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Appay R, Bielle F, Sievers P, Barets D, Fina F, Boutonnat J, Adam C, Gauchotte G, Godfraind C, Lhermitte B, Maurage C-A, Meyronet D, Mokhtari K, Rousseau A, Tauziède-Espariat A, Tortel M-C, Uro-Coste E, Burel-Vandenbos F, Chotard G, Pesce F, Varlet P, Colin C, Figarella-Branger D. Rosette-forming glioneuronal tumours are midline, FGFR1-mutated tumours. Neuropathol Appl Neurobiol. 2022;48:e12813. doi: 10.1111/nan.12813. - DOI - PubMed
-
- Appay R, Fina F, Barets D, Gallardo C, Nanni-Metellus I, Scavarda D, Henaff D, Vincent J, Grewis L, Pourquier P, Colin C, Figarella-Branger D. Multiplexed droplet digital PCR assays for the simultaneous screening of major genetic alterations in tumors of the central nervous system. Front Oncol. 2020;10:579762. doi: 10.3389/fonc.2020.579762. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

