Large-scale multimodal surface neural interfaces for primates
- PMID: 36647381
- PMCID: PMC9840154
- DOI: 10.1016/j.isci.2022.105866
Large-scale multimodal surface neural interfaces for primates
Abstract
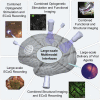
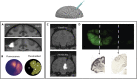
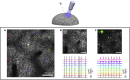
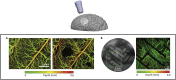
Deciphering the function of neural circuits can help with the understanding of brain function and treating neurological disorders. Progress toward this goal relies on the development of chronically stable neural interfaces capable of recording and modulating neural circuits with high spatial and temporal precision across large areas of the brain. Advanced innovations in designing high-density neural interfaces for small animal models have enabled breakthrough discoveries in neuroscience research. Developing similar neurotechnology for larger animal models such as nonhuman primates (NHPs) is critical to gain significant insights for translation to humans, yet still it remains elusive due to the challenges in design, fabrication, and system-level integration of such devices. This review focuses on implantable surface neural interfaces with electrical and optical functionalities with emphasis on the required technological features to realize scalable multimodal and chronically stable implants to address the unique challenges associated with nonhuman primate studies.
Keywords: Neuroscience; Optoelectronics.
© 2022 The Author(s).
Conflict of interest statement
The authors declare no competing interest.
Figures




References
-
- Fiáth R., Hofer K.T., Csikós V., Horváth D., Nánási T., Tóth K., Pothof F., Böhler C., Asplund M., Ruther P., et al. Long-term recording performance and biocompatibility of chronically implanted cylindrically-shaped, polymer-based neural interfaces. Biomed. Tech. 2018;63:301–315. doi: 10.1515/BMT-2017-0154/. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

