Safety and immunogenicity of SII-NVX-CoV2373 (COVID-19 vaccine) in adults in a phase 2/3, observer-blind, randomised, controlled study
- PMID: 36647543
- PMCID: PMC9833646
- DOI: 10.1016/j.lansea.2022.100139
Safety and immunogenicity of SII-NVX-CoV2373 (COVID-19 vaccine) in adults in a phase 2/3, observer-blind, randomised, controlled study
Abstract
Background: NVX-CoV2373, a Covid-19 vaccine was developed in the USA with ∼90% efficacy. The same vaccine is manufactured in India after technology transfer (called as SII-NVX-CoV2373), was evaluated in this phase 2/3 immuno-bridging study.
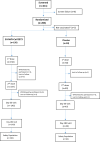
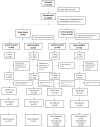
Methods: This was an observer-blind, randomised, phase 2/3 study in 1600 adults. In phase 2, 200 participants were randomized 3:1 to SII-NVX-CoV2373 or placebo. In phase 3, 1400 participants were randomized 3:1 to SII-NVX-CoV2373 or NVX-CoV2373 (940 safety cohort and 460 immunogenicity cohort). Two doses of study products (SII-NVX-CoV2373, NVX-CoV2373 or placebo) were given 3 weeks apart. Primary objectives were to demonstrate non-inferiority of SII-NVX-CoV2373 to NVX-CoV2373 in terms of geometric mean ELISA units (GMEU) ratio of anti-S IgG antibodies 14 days after the second dose (day 36) and to determine the incidence of causally related serious adverse events (SAEs) through 180 days after the first dose. Anti-S IgG response was assessed using an Enzyme-Linked Immunosorbent Assay (ELISA) and neutralizing antibodies (nAb) were assessed by a microneutralization assay using wild type SARS CoV-2 in participants from the immunogenicity cohort at baseline, day 22, day 36 and day 180. Cell mediated immune (CMI) response was assessed in a subset of 28 participants from immunogenicity cohort by ELISpot assay at baseline, day 36 and day 180. The total follow-up was for 6 months. Trial registration: CTRI/2021/02/031554.
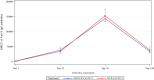
Findings: Total 1596 participants (200 in Phase 2 and 1396 in Phase 3) received the first dose. SII-NVX-CoV2373 was found non-inferior to NVX-CoV2373 (anti-S IgG antibodies GMEU ratio 0.91; 95% CI: 0.79, 1.06). At day 36, there was more than 58-fold rise in anti-S IgG and nAb titers compared to baseline in both the groups. On day 180 visit, these antibody titers declined to levels slightly lower than those after the first dose (13-22 fold-rise above baseline). Incidence of unsolicited and solicited AEs was similar between the SII-NVX-CoV2373 and NVX-CoV2373 groups. No adverse event of special interest (AESI) was reported. No causally related SAE was reported.
Interpretation: SII-NVX-CoV2373 induced a non-inferior immune response compared to NVX-CoV2373 and has acceptable safety profile.
Funding: SIIPL, Indian Council of Medical Research, Novavax.
Keywords: Immunogenicity; NVX-CoV2373; Non-inferiority; SII-NVX-CoV2373; Safety.
© 2022 The Author(s).
Conflict of interest statement
PSK, DK, BG, CB, AD, MG, and US are employees of SIIPL. JSP, MZ and SCC are employees of Novavax Inc. CSP is Chairman and Managing Director of SIIPL. All other authors declare no competing interests.
Figures

References
-
- World Health Organization (WHO) WHO Director-General’s opening remarks at the media briefing on COVID-19. 2020. https://www.who.int/director-general/speeches/detail/who-director-genera...
LinkOut - more resources
Full Text Sources
Miscellaneous

