Physical, Static, and Kinetic Analysis of the Electrochemical Deposition Process for the Recovery of Heavy Metal from Industrial Wastewater
- PMID: 36647551
- PMCID: PMC9840546
- DOI: 10.1155/2023/2741586
Physical, Static, and Kinetic Analysis of the Electrochemical Deposition Process for the Recovery of Heavy Metal from Industrial Wastewater
Abstract
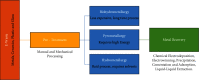
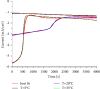
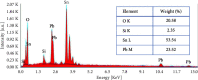
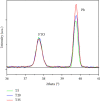
Through the electrodeposition technique, toxic metals in wastewater can be removed and deposited on a chosen substrate with excellent selectivity. In this work, we use this technique to extract lead cations from simulated wastewater by using fluorine-doped tin oxide (FTO) substrate at various temperatures. In situ tracking of lead nucleation at advanced stages has been achieved by chronoamperometry. According to the experimental results, the theoretical models developed to study the kinetic growth of lead deposits in 2D and 3D are in good agreement. Nucleation rate and growth rate constants, for example, were found to be strongly influenced by temperature. Cottrell's equation is used to calculate the diffusion coefficient. X-ray diffraction, scanning electron microscopy, and energy-dispersiveX-ray techniques were used to investigate and characterize the lead deposits. The reported results could provide insight into the optimization of electrodeposition processes for heavy metal recovery from wastewater and electronic wastes.
Copyright © 2023 Ridha Hamdi et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures




Similar articles
-
In Situ Electrodeposition of Pb and Ag Applied on Fluorine Doped Tin Oxide Substrates: Comparative Experimental and Theoretical Study.Materials (Basel). 2022 Dec 12;15(24):8865. doi: 10.3390/ma15248865. Materials (Basel). 2022. PMID: 36556671 Free PMC article.
-
Selective recovery of lead and zinc through controlling cathodic potential in a bioelectrochemically-assisted electrodeposition system.J Hazard Mater. 2020 Mar 15;386:121941. doi: 10.1016/j.jhazmat.2019.121941. Epub 2019 Dec 19. J Hazard Mater. 2020. PMID: 31884365
-
Potential Oscillated Electrochemical Metal Recovery System with Improved Conversion Kinetics and High Levelized Quality.Environ Sci Technol. 2021 Nov 16;55(22):15380-15389. doi: 10.1021/acs.est.1c03963. Epub 2021 Oct 28. Environ Sci Technol. 2021. PMID: 34709039
-
Upflow anaerobic sludge blanket reactor--a review.Indian J Environ Health. 2001 Apr;43(2):1-82. Indian J Environ Health. 2001. PMID: 12397675 Review.
-
Treatment of electroplating industry wastewater: a review on the various techniques.Environ Sci Pollut Res Int. 2022 Oct;29(48):72196-72246. doi: 10.1007/s11356-022-18643-y. Epub 2022 Jan 27. Environ Sci Pollut Res Int. 2022. PMID: 35084684 Review.
References
-
- Godswill C., Twinomuhwezi H. Industrial Waste Management, Treatment, and Health Issues: Wastewater, Solid, and Electronic Wastes . Chennai, India: Industrial Waste; 2020.
-
- Paździor K., Bilińska L., Ledakowicz S. A review of the existing and emerging technologies in the combination of AOPs and biological processes in industrial textile wastewater treatment. Chemical Engineering Journal . 2019;376 doi: 10.1016/j.cej.2018.12.057.120597 - DOI
LinkOut - more resources
Full Text Sources

