Efficacy and safety of ciprofol vs. propofol for the induction and maintenance of general anaesthesia: A multicentre, single-blind, randomised, parallel-group, phase 3 clinical trial
- PMID: 36647565
- PMCID: PMC10155686
- DOI: 10.1097/EJA.0000000000001799
Efficacy and safety of ciprofol vs. propofol for the induction and maintenance of general anaesthesia: A multicentre, single-blind, randomised, parallel-group, phase 3 clinical trial
Abstract
Background: HSK3486 (ciprofol) is a 2,6-disubstituted phenol derivative that acts like propofol as an agonist at the gamma-aminobutyric acid-A (GABA A ) receptor.
Objective: To investigate the efficacy and safety of HSK3486 for general anaesthesia induction and maintenance.
Design: A single-blinded, randomised, parallel-group, phase 3 trial.
Setting: Involving 10 study centres, from November 24, 2020 to January 25, 2021.
Patients: A total of 129 patients undergoing nonemergency, noncardiothoracic, and nonneurosurgical elective surgery.
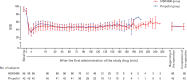
Intervention: Patients were randomly assigned at a 2:1 ratio into HSK3486 or propofol groups, to receive HSK3486 (0.4 mg kg -1 ) or propofol (2.0 mg kg -1 ) for induction before a maintenance infusion at initial rates of 0.8 and 5.0 mg kg -1 h -1 , and were adjusted to maintain a bispectral index (BIS) of 40-60 until the end of surgery.
Main outcome measures: Noninferiority between the drugs was evaluated as the lower limit of the 95% confidence interval (CI) for the between-group difference in the success rate of anesthetic maintenance (primary outcome) >-8%. Secondary outcomes included successful anaesthetic induction, full alertness and spontaneous breathing recovery, time until leaving the postanaesthesia care unit and changes in BIS. Safety profiles were also measured.
Results: Of 129 enrolled patients, 128 completed the trial, with 86 in the HSK3486 group and 42 in the propofol group. The success rate for the maintenance of general anaesthesia was 100% for both groups, and noninferiority of HSK3486 was confirmed (95% CI -4.28% to 8.38%). No significant differences were found between the two groups of patients with regard to secondary outcomes (all P > 0.05). There appeared to be a comparable incidence of treatment for emergency adverse events (TEAEs) (80.2% vs. 81.0%, P = 1.000) and drug-related TEAEs (57.0% vs. 64.3%, P = 0.451) in the HSK3486 and propofol groups.
Conclusion: HSK3486 had a noninferior efficacy profile compared to propofol, exhibiting excellent tolerance.
Trial registration: Clinicaltrials.gov, identifier: NCT04511728.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the European Society of Anaesthesiology and Intensive Care.
Figures
References
-
- Qin L, Ren L, Wan S, et al. . Design, synthesis, and evaluation of novel 2,6-disubstituted phenol derivatives as general anesthetics. J Med Chem 2017; 60:3606–3617. - PubMed
-
- Teng Y, Ou M, Wang X, et al. . Efficacy and safety of ciprofol for the sedation/anesthesia in patients undergoing colonoscopy: phase IIa and IIb multicenter clinical trials. Eur J Pharm Sci 2021; 164:105904. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous